Summary
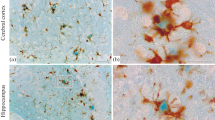
Peroxidase-labeled lectins were used for detection of specific monosaccharide residues in amyloid plaques in brains of scrapie-infected mice. The lectins tested recognize the following residues: β-d-galactosyl (Ricinus communis agglutinin 120, RCA-1), α-d-galactosyl and α-d-galactopyranoside (Bandeirea simplicifolia aggl., BSA), α-d-mannosyl and α-d-glucosyl (Concanavalin A, Con A), N-acetylglucosaminyl and sialyl (Wheat germ aggl., WGA), sialoglycoconjugates (Limulus polyphemus aggl., LPA), α-l-fucosyl (Ulex europeus aggl., UEA-1 and Tetragonolobus aggl., TPA), N-acetyl-d-galactosaminyl (Helix pomatia aggl., HPA). The most intense staining reaction in amyloid plaques was observed with BSA and WGA; it was less intense with RCA-1, Con A, and HPA. This indicates that the plaque material contains glycoproteins with abundance of accessible residues of α- and β-galactose, N-acetyl-d-glucosamine and N-actyl-d-galactosamine, and some types of sialoglycoconjugates recognized by WGA. Such residues, like α-l-flucosyl recognized by UEA-1 and TPA, were almost undectectable in the examined plaques.
There were also some differences in the staining intensity between small and large plaques (WGA and HPA) and between central and peripheral areas of the plaques.
In the wall of micro-blood vessels relatively strong staining reaction was observed with RCA and BSA and less intense with WGA and Con A.
Similar content being viewed by others
References
Bolton DC, McKinley MP and Prusiner SB (1982) Identification of a protein that purifies with the scrapie prion. Science 218:1309–1311
Bolton DC, Meyer RK, Prusiner SB (1985) Scrapie PrP27–30 is a sialoglycoprotein. J Virol 53:596–606
Bruce ME, Dickinson AG, Fraser H (1976) Cerebral amyloidosis in scrapie in the mouse. Effect of agent strain and mouse genotype. Neuropathol Appl Neurobiol 2:471–478
Bruce ME (1980) The neuropathology of scrapie in mice, with special reference to cerebral amyloidosis and agent stability (Submitted for the degree of Doctor of Philosophy) (Brain Research)
Christensen HE (1968) Improved histochemical staining techniques for mucopolysaccharides applied to experimental and human amyloids. In: Mandema E, Ruinen L, Scholten JH, Cohen AS (eds) Proceedings of the amyloidosis. Excerpta Medica, Amsterdam, pp 235–242
Cohen AS (1968) High resolution ultrastructure, immunology and biochemistry of amyloid. In: Amyloidosis. Excerpta Medica Foundation, pp 149–167
DeArmond SY, Kretzschmar HA, McKinley MP, Barry RA, Braunfeld MB and Prusiner SB (1985) Identification of prion amyloid filaments in scrapie-infected brain. Amer Assoc of Neuropath Abstr of 61st Ann Meeting, Boston June 1985, No. 171
DeArmond SJ, McKinley MP, Barry, RA, Braunfeld M, McColloch JR and Prusiner SB (1985) Identification of prion amyloid filaments in scrapie-infected brain. Cell 41:221–235
Graham RC, Karnovsky MJ (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of the mouse kidney: Ultrastructural cytochemistry by a new technique. J Histochem Cytochem 14:291–302
Holthöfer H, Virtanen T, Kariniemi AL, Hormia M, Linder E, Miettinen A (1982) Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Investigation 47:60–66
Li JJ, McAdam KPWJ, DeLellis RA, Pereira MEA (1984) Human amyloid P component: A circulating immunoregulatory lectin. IV. International Symposium on Amyloidosis, No. 20
Lucocq JM, Roth J (1984) Applications of immunocolloid in light microscopy. J Histochem Cytochem 32:1075–1083
Margolis G (1959) Senile cerebral disease. A critical survey of traditional concepts based upon observations with newer technics. Lab Invest 8:335–370
Merz PA, Wisniewski HM, Sommerville RA, Bobin SA, Masters CL and Iqbal K (1983) Ultrastructural morphology of amyloid fibrils from neuritic and amyloid plaques. Acta Neuropathol (Berl) 60:113–124
Moretz RC, Wisniewski HM, Lossinsky AS (1983) Pathogenesis of neuritic and amyloid plaques in scrapie — ultrastructural study of early changes in the cortical neuropil. In: Samuel D et al. (eds) Aging of the brain. Raven Press, New York, pp 61–79
Pena SDJ, Gordon BB, Karpati, G, Carpenter S (1981) Lectin histochemistry of human skeletal muscle. J Histochem Cytochem 29:542–549
Pino R (1984) Ultrastructural localization of lectin receptors on the bone marrow sinusoidal endothelium of the rat. Am J Anatomy 169:259–272
Schulte B, Spicer SS (1985) Histochemical methods for characterizing secretory and cell surface sialoglycoconjugates. J Histochem Cytochem 33:427–438
Simionescu M, Simionescu N, Palade GE (1982) Differentiated microdomains on the luminal surface of capillary endothelium: distribution of lectin receptors. J Cell Biol 94:406–413
Soda R, Tavassoli M (1983) Mapping of the bone marrow sinus endothelium with lectins and glycosylated ferritins: Identification of differentiated microdomains and their functional significance. J Ultrastruct Res 84:229–310
Windrum GM, Kramer H (1957) Some observations on the histochemical reactions of amyloid. Arch Pathol 63:373–378
Wisniewski HM, Terry RD (1973) Reexamination of the pathogenesis of the senile plaque. In: Zimmerman HM (ed) Progress in neuropathology, vol 2. Grune and Stratton, New York, pp 1–26
Wisniewski HM, Bruce ME, Fraser H (1975) Infections etiology of neuritic (senile) plaques in mice. Science 190:1108–1110
Wisniewski HM, Moretz RC, Lossinsky AS (1981) Evidence for induction of localized amyloid deposits and neuritic plaques by an infectious agent. Ann Neurol 10:517–522
Wisniewski HM, Lossinsky AS, Moretz RC, Vorbrodt AW, Lassman H, Carp RJ (1983) Increased blood-brain barrier permeability in scrapie-infected mice. J Neuropathol Exp Neurol 43:615–626
Wisniewski HM, Merz GS (1985) Neuropathology of the aging brain and dementia of the Alzheimer type. Reprint from: Gaitz CM, Samorajski T (eds) Aging 2000: Our health care destiny, vol I: Biomedical Issues, chapt 20. Springer, New York, pp 231–243
Yamada K, Shimizu S (1979) The use of peroxidase-labeled Limulus polyphemus agglutinin for the histochemistry of sialic acid-containing glycoproteins in light microscopy. Histochem J 11:457–471
Author information
Authors and Affiliations
Additional information
Support in part by grant no. 5PO1 AG 04220-03 from the National Institute of Aging, NIH
Rights and permissions
About this article
Cite this article
Szumanska, G., Vorbrodt, A.W. & Wisniewski, H.M. Lectin histochemistry of scrapie amyloid plaques. Acta Neuropathol 69, 205–212 (1986). https://doi.org/10.1007/BF00688295
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00688295