Abstract
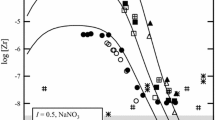
The temperature dependencies of europium carbonate stability constants were examined at 15, 25, and 35°C in 0.68 molal Na+(ClO −4 , HCO −3 ) using a tributyl phosphate solvent extration technique. Our distribution data can be explained by the equilibria
Similar content being viewed by others
References
L. V. Ruzaikina, I. N. Marov, V. A. Ryabukhin, A. N. Ermakov, and V. N. Filimonova,Zh. Anal. Khim. 33, 1082 (1978).
D. Ferri, I. Grenthe, S. Hietanen, and F. Salvatore,Acta Chem. Scand. A37, 359 (1983).
L. Ciavatta, D. Ferri, I. Grenthe, F. Salvatore, and K. Spahiu,Acta Chem. Scand. A35, 403 (1981).
R. Lundqvist,Acta Chem. Scand. A36, 741 (1982).
K. J. Cantrell, Thesis, University of South Florida, (1986).
R. Lundqvist, J. Lu, and I. Svantesson,Acta Chem. Scand. A38, 501 (1984).
D. A. Orth, R. M. Wallace, and D. G. Karraker, in ‘Solvent Extraction Reactions and Mechanism’Science and Technology of Tributyl Phosphate Vol. 1, W. W. Schultz, J. D. Navratil, and A. E. Talbot, eds., (CRC Press, 1984), Chap. 6.
P. G. Manning,Can. J. Chem. 43, 2911 (1965).
W. A. E. McBryde,Analyst 94, 337 (1969).
W. A. E. McBryde,Analyst 96, 739 (1971).
R. H. Byrne and D. R. Kester,J. Solution Chem. 7, 373 (1978).
D. W. Marquardt,J. Soc. Ind. Appl. Math 11, 431 (1963).
A. Barr, J. Goodnight, J. Sall, and J. Helwig,A User's Guide to SAS 76, (SAS Institute, Raleigh, 1976).
M. Frydman, G. Nilsson, T. Rengemo, and L. G. Sillen,Acta Chem. Scand. 12, 878 (1958).
R. H. Byme and W. L. Miller,Geochim. Cosmochim. Acta 49, 1837 (1985).
V. Thurmond and F. J. Millero,J. Solution Chem. 11 (7), 447 (1982).
L. G. Sillen and A. E. Martell,Stability Constants of Metal-Ion complexes, Special Publication No. 17, The Chemical Society (Burlington House, 1964).
L. G. Sillen and A. E. Martell,Stability Constants of Metal-Ion Complexes, Special Publication No. 25, The Chemical Society (Burlington House, 1971).
W. Stumm and J. J. Morgan,Aquatic Chemistry, (Wiley, 1981).
F. J. Millero and D. R. Schreiber,Amer. J. Sci. 282, 1508 (1982).
A. E. Martell and R. M. Smith,Critical Stability Constants, Vol. 3, (Plenum, 1977).
Y. S. Sklyarenko, L. V. Ruzaikina,Russ. J. Inorg. Chem. 15, (3), 339 (1970).
D. Ferri and F. Salvatore,Acta Chem. Scand. A37, 531 (1983).
C. F. Baes and R. E. Mesmer, in ‘Yttrium, the Lanthanides, and Actinium’,The Hydrolysis of Cations, (Wiley, 1976), Chap. 7.
F. Kepak,Chem. Rev. 71 (4), 357 (1971).
K. Spahiu,Acta Chem. Scand. A39 33 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cantrell, K.J., Byrne, R.H. Temperature dependence of europium carbonate complexation. J Solution Chem 16, 555–566 (1987). https://doi.org/10.1007/BF00646333
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00646333