Abstract
The solubility of amorphous zirconium hydroxide [Zr(OH)4(am)] was investigated in carbonate solutions containing various concentrations of sodium nitrate. The observed dependences of Zr(IV) solubility on the hydrogen ion concentration (pHc) and carbonate concentration suggested the formation of \( {\text{Zr}}({{\text{CO}}}_{3} )_{4}^{4 - } \), \( {\text{Zr}}({{\text{CO}}}_{3} )_{5}^{6 - } \), and \( {\text{Zr(OH)}}_{ 2} ( {{\text{CO}}}_{3} )_{2}^{2 - } \) as the dominant species in the neutral to weakly alkaline pH regions. The solubility of Zr(IV) at certain pHc values and carbonate concentrations was observed to increase slightly with increasing ionic strength, while the solid phase was determined to be Zr(OH)4(am) at all ionic strengths by using thermal analysis. By applying the specific ion interaction theory, the solubility data at different pHcs, carbonate concentrations, and ionic strengths were analyzed to determine the formation constants of the Zr(IV) carbonate complexes and their ion interaction coefficients. The obtained values explain well the solubility data, which are discussed in comparison with those of analogous tetravalent actinide carbonates.






Similar content being viewed by others
References
Guillaumont, R., Fanghänel, T., Fuger, J., Grenthe, I., Neck, V., Palmer, D., Rand, M.: Update on the chemical thermodynamics of uranium, neptunium, plutonium, americium and technetium. In: Mompean, F.J., Illemassène, M., Domenech-Orti, C., Ben Said, K. (eds.) Chemical Thermodynamics, vol. 5. Elsevier, Amsterdam (2003)
Brown, P., Curti, E., Grambow, B., Ekberg, C.: Chemical thermodynamics of zirconium. In: Mompean, F.J., Perrone, J., Ekberg, C. (eds.) Chemical Thermodynamics, vol. 8. Elsevier, Amsterdam (2005)
Rand, M., Fuger, J., Grenthe, I., Neck, V., Rai, D.: Chemical thermodynamics of thorium. In: Mompean, F.J., Perrone, J., Illemaassène, M. (eds.) Chemical Thermodynamics, vol. 11. Elsevier, Amsterdam (2009)
Fanghänel, Th, Neck, V.: Aquatic chemistry and solubility phenomena of actinide oxides/hydroxides. Pure Appl. Chem. 74, 1895–1907 (2002)
Altmaier, M., Gaona, X., Fanghänel, T.: Recent advances in aqueous actinide chemistry and thermodynamics. Chem. Rev. 113, 901–943 (2013)
Knope, K.E., Soderholm, L.: Solution and solid-state structural chemistry of actinide hydrates and their hydrolysis and condensation products. Chem. Rev. 113, 944–994 (2013)
Dervin, J.: Sur la structure et la stabilité des carbonates complexes de Thorium(IV), Cerium(IV), Zirconium(IV), Hafnium(IV). Ph.D. Thesis, University of Reims Champagne-Ardenne (1972)
Malinko, L.A., Chumakova, L.S., Arsenin, K.I., Karlysheva, K.F., Sheka, I.A.: The interaction of zirconium sulphate with sodium carbonate in solution. Russ. J. Inorg. Chem. 25, 1184–1188 (1980)
Karlysheva, K.F., Chumakova, L.S., Malinko, L.A., Sheka, I.A.: Reaction of zirconium and hafnium oxide chlorides with sodium carbonate in solution. Russ. J. Inorg. Chem. 27, 1582–1585 (1982)
João, A., Bigot, S., Fromage, F.: Etude des carbonates complexes des éléments IVB II. Détermination des constants d’équilibre de formation des tétracarbonatozirconates(IV) et -hafnates(IV). Bull. Soc. Chim. Fr. 6, 943–947 (1987)
Veyland, A., Rimbault, J., Dupont, L., Pierrard, J.C., Aplincourt, M., Bourg, S., Nuzillard, J.M., Angiboust, J.F.: Coordination mode and kinetic behavior of the tetracarbonatozirconate ion. Helv. Chim. Acta 82, 2003–2014 (1999)
Veyland, A., Dupont, L., Rimbault, J., Pierrard, J.C., Aplincourt, M.: Aqueous chemistry of zirconium(IV) in carbonate media. Helv. Chim. Acta 83, 414–427 (2000)
Veyland, A., Dupont, L., Pierrard, J.C., Rimbault, J., Aplincourt, M., Devoldere, L.: Investigation of the tetracarbonatozirconate ion by electrospray mass spectrometry in aqueous media. Inorg. Chem. Commun. 3, 600–607 (2000)
Pouchon, M.A., Curti, E., Degueldre, C., Tobler, L.: The influence of carbonate complexes on the solubility of zirconia: new experimental data. Prog. Nucl. Energy 38, 443–446 (2001)
Japan Atomic Energy Agency: 2nd Progress Report on Research and Development for TRU Waste Disposal in Japan, JAEA-Review TRU-TR2-2007-01, JAEA, Tokai, Japan (2007)
Rudisell, T.S.: Decontamination of zircaloy cladding hulls from spent nuclear fuel. J. Nucl. Fuels 385, 193–195 (2009)
Reynolds, J.G., Huber, H.J., Cooke, G.A., Pestovich, J.A.: Solid-phase zirconium and fluoride species in alkaline zircaloy cladding waste at Hanford. J. Hazard. Mater. 278, 203–210 (2014)
Sasaki, T., Kobayashi, T., Takagi, I., Moriyama, H.: Solubility measurement of zirconium(IV) hydrous oxide. Radiochim. Acta 94, 489–494 (2006)
Altmaier, M., Neck, V., Fanghänel, T.: Solubility of Zr(IV), Th(IV) and Pu(IV) hydrous oxides in CaCl2 solutions and the formation of ternary Ca–M(IV)–OH complexes. Radiochim. Acta 96, 541–550 (2008)
Kobayashi, T., Sasaki, T., Takagi, I., Moriyama, H.: Zirconium solubility in ternary aqueous system of Zr(IV)–OH–carboxylates. J. Nucl. Sci. Technol. 46, 142–148 (2009)
Altmaier, M., Neck, V., Müller, R., Fanghänel, T.: Solubility of ThO2·xH2O(am) in carbonate solution and the formation of ternary Th(IV) hydroxide–carbonate complexes. Radiochim. Acta 93, 83–92 (2005)
Altmaier, M., Neck, V., Denecke, M.A., Yin, R., Fanghänel, T.: Solubility of ThO2·xH2O(am) and the formation of ternary Th(IV) hydroxide–carbonate complexes in NaHCO3–Na2CO3 solutions containing 0–4 M NaCl. Radiochim. Acta 94, 495–500 (2006)
Rai, D., Felmy, A.R., Hess, N.J., Moore, D.A., Yui, M.: A thermodynamic model for the solubility of UO2(am) in the aqueous K+–Na+–\( {\text{HCO}}_{ 3}^{-}\)–\( {{\text{CO}}}_{3}^{2 - } \)–OH−–H2O system. Radiochim. Acta 82, 17–25 (1998)
Rai, D., Hess, N.J., Felmy, A.R., Moore, D.A.: A thermodynamic model for the solubility of NpO2(am) in the aqueous K+–\( {\text{HCO}}_{ 3}^{-}\)–\( {{\text{CO}}}_{3}^{2 - } \)–OH−–H2O system. Radiochim. Acta 84, 159–169 (1999)
Rai, D., Hess, N.J., Felmy, A.R., Moore, D.A., Yui, M., Vitorge, P.: A thermodynamic model for the solubility of PuO2(am) in the aqueous K+–\( {\text{HCO}}_{ 3}^{-}\)–\( {{\text{CO}}}_{3}^{2 - } \)–OH−–H2O system. Radiochim. Acta 86, 89–99 (1999)
Östhols, E., Bruno, J., Grenthe, I.: On the influence of carbonate on mineral dissolution: III. The solubility of microcrystalline ThO2 in CO2–H2O media. Geochim. Cosmochim. Acta 58, 613–623 (1994)
Sasaki, T., Kobayashi, T., Takagi, I., Moriyama, H.: Hydrolysis constant and coordination geometry of zirconium(IV). J. Nucl. Sci. Technol. 45, 735–739 (2008)
Capdevila, H., Vitorge, P., Giffaut, E., Delmau, L.: Spectrophotometric study of the dissociation of the Pu(IV) carbonate limiting complex. Radiochim. Acta 74, 93–98 (1996)
Acknowledgements
This work has been supported in part by a grant from the Ministry of Economy, Trade and Industry (METI). The authors would like to acknowledge and thank Drs. Xavier Gaona and Marcus Altmaier of the Karlsruhe Institute of Technology for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
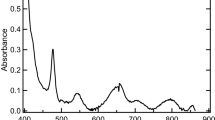
In the present study, the sample solutions were prepared using an undersaturation method. The initial solid phase of Zr(OH)4(am) was placed into the sample solutions at specific pH, [C]tot, and ionic strength. After aging of the sample solutions, the solid phases kept at pHc 8, [C]tot = 0.04 mol·dm−3, and I = (0.1, 0.5, 2.0 and 5.0) mol·dm−3 were investigated by thermal analysis. The TGA and DTA curves are shown in Fig. 7, together with those of Zr(OH)4(am), NaNO3, and NaHCO3. In the curves for NaNO3, an endothermic peak around 310 °C represents the melting point, while the weight loss after 650 °C indicates thermal decomposition. For NaHCO3, an endothermic peak and weight loss at 150 °C shows a release of CO2 from the sample. For Zr(OH)4(am), an endothermic peak and weight loss at around 100 °C represent the dehydration of Zr(OH)4(am) to ZrO2(s). The solid phases that were in contact with the sample solutions showed endothermic peaks and weight losses corresponding only to the processes of dehydration of Zr(OH)4(am) and melting and decomposition of residual NaNO3. No indications of a Zr(IV) carbonate solid compound were observed, so it was assumed that the initial Zr(OH)4(am) solid phase was not transformed under the experimental conditions employed.
See Fig. 7.
Appendix 2: Experimental Solubility Data
Rights and permissions
About this article
Cite this article
Kobayashi, T., Sasaki, T. Solubility of Zr(OH)4(am) and the Formation of Zr(IV) Carbonate Complexes in Carbonate Solutions Containing 0.1–5.0 mol·dm−3 NaNO3 . J Solution Chem 46, 1741–1759 (2017). https://doi.org/10.1007/s10953-017-0599-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-017-0599-6