Abstract
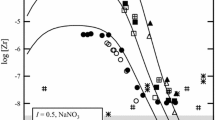
The solubilities of NiCO3, ZnCO3, and CdCO3 in NaClO4–NaHCO3–Na2CO3 solutions are studied experimentally at 25°C and ionic strength of 0.1. The resulting data are used to determine the concentration stability constants of NiCO3 0, ZnCO3 0, and CdCO3 0 complexes in aqueous solutions (they are equal to 810, 12700, and 17900, respectively; log \(K'_{MCO_3^0 } \) = 2.90 ± 0.14, 4.10 ± 0.20, and 4.25 ± 0.04) and to calculate their thermodynamic values (6.45 × 103, 1.01 × 105, and 1.43 × 105, respectively). The logarithms of stability constants of carbonate complexes are found to be inversely dependent on the solubility products of their metal carbonates.
Similar content being viewed by others
References
L. G. Sillen and A. E. Martell, The Chemical Society Special Publications, No. 17: Stability Constants of Metal–Ion Complexes (Burlington House, London, 1964).
G. B. Naumov, B. N. Ryzhenko, and I. L. Khodakovskii, The Handbook of Thermodynamic Data, Ed. by A. I. Tugarinov (Atomizdat, Moscow, 1971) [in Russian].
R. M. Smith and A. E. Martell, Critical Stability Constants (Plenum, New York, 1976).
G. A. Volkov and G. A. Solomin, Gidrogeol. Inzh. Geol.. Ekspress-Inf., No. 3, 1 (1983).
F. J. Millero, Physical Chemistry of Natural Waters (Willey-Interscience, New York et al., 2001).
V. Gutmann, Coordination Chemistry in Non-Aqueous Solutions (Springer, Wien, 1968).
V. N. Kumok, O. M. Kuleshova, and L. A. Karabin, The Solubility Product (Nauka SO, Novosibirsk, 1983) [in Russian].
A. E. Martell, R. M. Smith, and R. J. Motekaitis, Critically Selected Stability Constants of Metal Complexes Database. Version 3.0 (Texas A&M Univ. College Station, TX. 1997).
V. S. Savenko and A. V. Savenko, Russ. J. Inorg. Chem. 42, 1776 (1997).
A. V. Savenko, Russ. J. Inorg. Chem. 46, 1102 (2001).
V. S. Savenko, Russ. J. Inorg. Chem. 43, 461 (1998).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.V. Savenko, V.S. Savenko, 2017, published in Zhurnal Neorganicheskoi Khimii, 2017, Vol. 62, No. 5, pp. 685–688.
Rights and permissions
About this article
Cite this article
Savenko, A.V., Savenko, V.S. Determination of stability constants of the NiCO3 0, ZnCO3 0, and CdCO3 0 complexes using solubilities of corresponding metal carbonates in aqueous solutions. Russ. J. Inorg. Chem. 62, 679–682 (2017). https://doi.org/10.1134/S0036023617050205
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023617050205