Summary
-
1.
The response properties of 322 single units in the electroreceptive midbrain (lateral mesencephalic nucleus, LMN) of the thornback ray,Platyrhinoidis triseriata, were studied using uniform and local electric fields. Tactile, visual, or auditory stimuli were also presented to test for multimodality.
-
2.
Most LMN electrosensory units (81%) are silent in the absence of stimulation. Those with spontaneous activity fired irregularly at 0.5 to 5 impulses/s, the lower values being more common. Two units had firing rates greater than 10/s.
-
3.
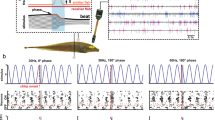
Midbrain electrosensory units are largely phasic, responding with one or a few spikes per stimulus onset or offset or both, but the adaptation characteristics of some neurons are complex. The same neuron can exhibit phasic or phasic-tonic responses, depending upon orientation of the electric field. Tonic units without any initial phasic overshoot were not recorded. Even the phasic-tonic units adapt to a step stimulus within several seconds.
-
4.
Unit thresholds are generally lower than 0.3 μV/cm, the weakest stimulus delivered, although thresholds as high as 5μV/cm were recorded. Neuronal responses reach a maximum, with few exceptions, at 100 μV/cm and decrease rapidly at higher intensities.
-
5.
LMN neurons are highly sensitive to stimulus repetition rates: most responded to frequencies of 5 pulses/s or less: none responded to rates greater than 10/s. Three distinct response patterns are recognized.
-
6.
Best frequencies in response to sinusoidal stimuli range from 0.2 Hz (the lowest frequency delivered) to 4 Hz. Responses decrease rapidly at 8 Hz or greater, and no units responded to frequencies greater than 32 Hz.
-
7.
Most LMN neurons have small, well defined excitatory electroreceptive fields (RFs) exhibiting no surround inhibition, at least as detectable by methods employed here. Seventy-eight percent of units recorded had RFs restricted to the ventral surface: of these, 98% were contralateral. The remaining 22% of units had disjunct dorsal and ventral receptive fields.
-
8.
Electrosensory RFs on the ventral surface are somatotopically organized. Anterior, middle, and posterior body surfaces are mapped at the rostral, middle, and caudal levels, respectively, of the contralateral LMN. The lateral, middle, and medial body are mapped at medial, middle, and lateral levels of the nucleus. Moreover, the RFs of all units isolated in a given dorsoventral electrode track are nearly superimposable. About 40% of LMN, measured from the dorsal surface, is devoted to input from ventral electroreceptors located in a small region rostral and lateral to the mouth.
-
9.
The rostrocaudal extent of the somatotopic map includes regions of the neuraxis previously divided into 2 nuclei of the lateral mesencephalic complex, the anterior nucleus (AN) anteriorly and lateral nucleus (LMN) caudally. The single, unfractured somatotopic map reported here is continuous across the boundary of these two regions. This suggests that AN may not be a distinct nucleus but rather a rostral extension of LMN.
-
10.
LMN neurons are sensitive to the orientation of a uniform electric field. Maximum and minimum responses can be recorded at orientations separated by just 30°. Single unit best orientations are largely a function of the orientation of ampullary canals from which a unit receives input.
-
11.
The majority of LMN electrosensory units are bimodal, responding vigorously to a camel-hair brush applied gently to the skin. This midbrain mechanosensitivity apparently is not mediated by the ampullae of Lorenzini since tactile stimuli adequate in the midbrain do not excite medullary electrosensory neurons. Mechanosensory minimum RFs are generally larger than but invariably overlap with electrosensory RFs. The somatotopic maps of the 2 modalities are congruent. Cross-modality interactions in bimodal units were observed and will be discussed.
Similar content being viewed by others
Abbreviations
- DON :
-
dorsal octavolateralis nucleus
- LMN :
-
lateral mesencephalic nucleus
- PSTH :
-
poststimulus time histogram
- RF :
-
receptive field
References
Akoev GN, Ilyinsky OB (1973) Some functional characteristics of the electroreceptors (the ampullae of Lorenzini) of elasmobranchs. Experientia 29:293–294
Akoev GN, Ilyinsky OB, Zadan PM (1976a) Physiological properties of electroreceptors of marine skates. Comp Biochem Physiol A 53:201–209
Akoev GN, Ilyinsky OB, Zadan PM (1976b) Responses of electroreceptors (ampullae of Lorenzini) of skates to electric and magnetic fields. J Comp Physiol 106:127–136
Andrianov GN, Brown HR, Ilyinsky OB (1974) Responses of central neurons to electrical and magnetic stimuli of the ampullae of Lorenzini in the Black Sea Skate. J Comp Physiol 93:287–299
Andrianov YuN, Broun GR, Ilynsky OB, Muraveiko VM (1984) Central projections of ampullae of Lorenzini in skates. Neurophysiology 15:451–457
Barlow HB (1953) Summation and inhibition in the frog's retina. J Physiol 119:69–88
Bastian J (1982) Vision and electroreception: integration of sensory information in the optic tectum of the weakly electric fishApteronotus albifrons. J Comp Physiol 147:287–297
Bodznick DA, Northcutt RG (1980) Segregation of electro- and mechanoreceptive inputs to the elasmobranch medulla. Brain Res 195:313–322
Bodznick D, Northcutt RG (1984) An electrosensory area in the telencephalon of the little skate,Raja erinacea. Brain Res 298:117–124
Bodznick D, Schmidt AW (1984) Somatotopy within the medullary electrosensory nucleus of the little skate,Raja erinacea. J Comp Neurol 225:581–590
Boord RF, Northcutt RG (1982) Ascending lateral line pathways to the midbrain of the clearnose skate,Raja eglanteria. J Comp Neurol 207:274–282
Bretschneider F, Peters RC, Peele PH, Dorresteijn A (1980) Functioning of catfish electroreceptors: statistical distribution of sensitivity and fluctuations of spontaneous activity. J Comp Physiol. 137:273–279
Bretschneider F, Weille JR de, Klis JFL (1985) Functioning of catfish electroreceptors: fractional-order filtering and non-linearity. Comp Biochem Physiol 80A: 191–198
Bromm B, Hensel H, Tagmat AT (1976) The electrosensitivity of the isolated ampulla of Lorenzini in the dogfish. J Comp Physiol 111: 127–136
Broun GR, Ilyinsky OB, Volkova NK (1972) The study of some properties of electroreceptor structures of the lateral line in skates. Fiziol Zh SSSR im IM Sechenova 58:1499–1505
Brown HR, Ilyinsky OB (1978) The ampullae of Lorenzini in the magnetic field. J Comp Physiol 126:333–341
Bullock TH (1979) Processing of ampullary input in the brain: comparison of sensitivity and evoked responses among elasmobranchs and siluriform fishes. J Physiol (Paris) 75:297–407
Bullock TH (1981) Comparisons of the acoustic and electric senses and their central processing. In: Fay RR, Popper AN, Tavolga WN (eds) Hearing and sound communication in fishes. Springer, Berlin Heidelberg New York, pp 525–572
Bullock TH (1984) Comparative neuroscience holds promise for quiet revolutions. Science 225:473–478
Bullock TH, Corwin JT (1979) Acoustic evoked activity in the brain of sharks. J Comp Physiol 129:223–234
Butsuk SV, Bessonov BI (1981) Direct current electric field in some teleost species: effect of medium salinity. J Comp Physiol 141:277–282
Carr CE, Maler L, Heiligenberg W, Sas E (1981) Laminar organization of the afferent and efferent systems of the torus semicircularis of gymnotiform fish: morphological substrates for parallel processing in the electrosensory system. J Comp Neurol 203:649–670
Clarke PGH, Whitteridge D (1976) The projection of the retina, including the ‘red area’ onto the optic tectum of the pigeon. Q J Exp Physiol 61:351–358
Corwin JT, Northcutt RG (1982) Auditory centers in the elasmobranch brainstem: deoxyglucose autoradiography and evoked potential recording. Brain Res 236:261–273
De Weille JR (1983) Electrosensory information processing by lateral-line lobe neurons of catfish investigated by means of white noise cross-correlation. Comp Biochem Physiol 74A: 677–680
Dowben R, Rose JE (1953) A metal-filled microelectrode. Science 118:22824
Dykes RW (1983) Parallel processing of somatosensory information: a theory. Brain Res Rev 6:47–115
Ebbesson SOE, Hodde KC (1981) Ascending spinal systems in the nurse shark,Ginglyomostoma cirratum. Cell Tissue Res 216:313–331
Finger T (1978) Gustatory pathways in the bullhead catfish. II. Facial lobe connections. J Comp Neurol 180:591–706
Gordon BG (1973) Receptive fields in deep layers of cat superior colliculus. J Neurophysiol 36:157–178
Hartline HK (1938) The response of single optic nerve fibers of the vertebrate eye to illumination of the retina. Am J Physiol 121:400–415
Hartline P, Kaas L, Loop MS (1978) Merging of modalities in the optic tectum: infrared and visual integration in rattlesnakes. Science 199:1225–1229
Ingle D, Sprague JM (1975) Sensorimotor function of the midbrain tectum. Neurosci Res Prog Bull 13:169–288
Jones EG (1981) Functional subdivisions and synaptic organization of the mammalian thalamus. In: Porter R (ed) Neurophysiology IV. Int Rev Physiol 25. University Park Press, Baltimore, pp 173–243
Kalmijn AJ (1971) The electric sense of sharks and rays. J Exp Biol 55:371–383
Kalmijn AJ (1972) Bioelectric fields in sea water and the function of the ampullae of Lorenzini in elasmobranch fishes. Scripps Institution of Oceanography Ref Ser 72L-83:1–22
Kalmijn AJ (1974) The detection of electric fields from inanimate and animate sources other than electric organs. In: Fessard A (ed) Electroreceptors and other specialized receptors in vertebrates. (Handbook of sensory physiology, vol II/3). Springer, Berlin Heidelberg New York, pp 147–200
Kalmijn AJ (1977) The electric and magnetic sense of sharks, skates, and rays. Oceanus 20:45–52
Kalmijn AJ (1982) Electric and magnetic field detection in elasmobranch fishes. Science 218:916–917
Kalmijn AJ (1985) Theory of electromagnetic orientation: a further analysis. In: Bolis L, Keynes RD, Maddrell SHP (eds) Comparative physiology of sensory systems. Press Syndicate, University of Cambridge, Cambridge, pp 525–563
Knudsen EI (1976a) Midbrain responses to electroreceptive input in catfish. Evidence of orientation preferences and somatotopic organization. J Comp Physiol 106:51–67
Knudsen EI (1976b) Midbrain units in catfish: response properties to electroreceptive input. J Comp Physiol 109:315–335
Knudsen EI (1977) Distinct auditory and lateral line nuclei in the midbrain of catfishes. J Comp Neurol 173:417–432
Knudsen EI (1978) Functional organization in electroreceptive midbrain of the catfish. J Neurophysiol 41:350–364
Lettvin JY, Maturana HR, McCulloch WS, Pitts WH (1959) What the frog's eye tells the frog's brain. Proc Inst Radio Engr 47:1940–1951
Loewenstein WR (1960) Mechanisms of nerve impulse initiation in a pressure receptor (Lorenzinian Ampulla). Nature 188:1034–1035
Maturana HR, Lettvin JY, Pitts WH, McCulloch WS (1960) Physiology and anatomy of vision in the frog. J Gen Physiol 43:129–175
McCreery DB (1977) Two types of electroreceptive lateral lemniscal neurons of the lateral line lobe of the catfish,Ictalurus nebulosus; connections from the lateral line nerve and steady-state frequency response characteristics. J Comp Physiol 113:317–339
Montgomery JC (1984a) Frequency response characteristics of primary and secondary neurons in the electrosensory system of the thornback ray. Comp Biochem Physiol 79A: 189–195
Montgomery JC (1984b) Noise cancellation in the electrosensory system of the thornback ray; common mode rejection of input produced by the animal's own ventilatory movement. J Comp Physiol A 155:103–111
Mountcastle VB (1980) Sensory receptors and neuronal encoding: introduction to sensory processes. In: Mountcastle VB (ed) Medical physiology I ch 11. Mosby Co, St. Louis, pp 327–347
Murray RW (1960) The response of the ampullae of Lorenzini of elasmobranchs to mechanical stimulation. J Exp Biol 37:417–424
Newman EA, Hartline P (1981) Integration of visual and infrared information in bimodal neurons of the rattlesnake optic tectum. Science 213:789–791
Northcutt RG (1978) Brain organization in the cartilaginous fishes. In: Hodgson ES, Mathewson RF (eds) Sensory biology of sharks, skates, and rays. Office of Naval Research, Arlington, Va, pp 117–193
Penfield W, Rasmussen T (1950) The cerebral cortex of man. Macmillan, New York
Peters RC, Bretschneider F (1972) Electric phenomena in the habitat of the catfishIctalurus nebulosus. J Comp Physiol 81:345–362
Peters RC, Buwalda RJA (1972) Frequency responses of the electroreceptors (‘small pit organs’) of the catfish,Ictalurus nebulosus Les. J Comp Physiol 79:29–38
Peters RC, Loos WJG, Gerritsen A (1974) Distribution of electroreceptors, bioelectric field patterns, and skin resistance in the catfish,Ictalurus nebulosus LeS. J Comp Physiol 92:11–22
Peters RC, Wijland, F van (1974) Electro-orientation in the passive electric catfish,Ictalurus nebulosus LeS. J Comp Physiol 92:273–280
Platt CJ, Bullock TH, Czeh G, Kovacevic N, Konjevic DJ, Gojkovic M (1974) Comparison of electroreceptor, mechanoreceptor, and optic evoked potentials in the brain of some rays and sharks. J Comp Physiol 95:323–355
Roth A (1972) Wozu dienen die Elektrorezeptoren der Welse? J Comp Physiol 79:113–135
Roth A (1973) Ampullary electroreceptors in catfish: afferent fiber activity before and after removal of the sensory cells. J Comp Physiol 87:259–275
Roth A (1975) Central neurons involved in the electroreception of the catfishKryptopterus. J Comp Physiol 100:135–146
Schweitzer J (1983) The physiological and anatomical localization of two electroreceptive diencephalic nuclei in the thornback ray,Platyrhinoidis triseriata. J Comp Physiol 153:331–341
Schweitzer J, Lowe D (1984) Mesencephalic and diencephalic cobalt-lysine injections in an elasmobranch: evidence for two parallel electrosensory pathways. Neurosci Lett 44:317–322
Skarf B, Jones MJ (1981) Vestibular-visual interactions in frog mesencephalon during natural stimulation. Brain Res 206:457–461
Stein BE, Magalhaes-Castro B, Kruger L (1976) Relationship between visual and tactile representations in cat superior colliculus. J Neurophysiol 39:401–419
Stone J (1983) Parallel processing in the visual system. Plenum Press, New York London
Suga N (1967) Electrosensitivity of specialized and ordinary lateral line organs of the electric fish,Gymnotus carapo. In: Cahn P (ed) Lateral line detectors. Indiana University Press, Bloomington, pp 395–409
Terashima S, Goris RC (1975) Tectal organization of pit viper infrared reception. Brain Res 83:490–494
Tong S-L, Bullock TH (1982) The sensory functions of the cerebellum of the thornback ray,Platyrhinoidis triseriata. J Comp Physiol 148:399–410
Tusa RJ, Palmer LA, Rosenquist AC (1978) The retinotopic organization of area 17 (striate cortex) in the cat. J Comp Neurol 177:213–236
Ulinski PS (1984) Design features in vertebrate sensory systems. Am Zool 24:717–731
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schweitzer, J. Functional organization of the electroreceptive midbrain in an elasmobranch (Platyrhinoidis triseriata). J. Comp. Physiol. 158, 43–58 (1986). https://doi.org/10.1007/BF00614519
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00614519