Summary
-
1.
Ascending abdominal interneurons receiving inputs from the cerci were examined by stimulating the cerci with pulses of 30-Hz sound, a frequency that corresponds to the repetition rate of the syllables in the conspecific calling song.
-
2.
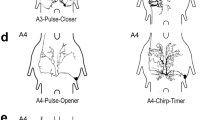
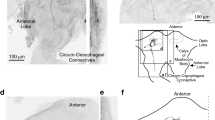
13 interneurons were morphologically identified and characterized physiologically.
-
3.
Neurons showing little or no habituation during the sound pulse copy the stimulus pattern by discharging at a particular phase of each cycle of air motion (interneurons 10-2a, 10-3a; with qualifications also 8-1a, 9-1a and 9-3a).
-
4.
Neurons in a second group act as frequency-dependent filters: three (9-1b, 10-1c and 11-1c) showed low-pass properties at 30 Hz, and one (8-1b) a band pass characteristic.
-
5.
The direction of the stimulus source also affects the response; individual neurons have different best directions. In principle the animal could determine the direction of air-particle oscillation by comparing the response phases of two cells (10-2a and 10-3a) that are shown to discharge in synchrony or in alternation depending on the direction of the stimulus.
-
6.
Changes of various parameters of the sound pulses during continuous stimulation of the cerci cause interneuron 11-1c and the newly described interneuron NN1 to give persistently increased responses.
Similar content being viewed by others
Abbreviations
- IN :
-
abdominal ascending interneuron
- I1–3 :
-
intensity 1–3
References
Belosky DC, Delcomyn F (1977) Information processing in a cricket ganglion: the response of giant fibres to sound pulses. J Insect Physiol 23:359–365
Bennet-Clark HC (1984) A particle velocity microphone for the song of small insects and other acoustic measurements. J Exp Biol 108:459–463
Camhi JM, Nolen TG (1981) Properties of the escape system of cockroaches during walking. J Comp Physiol 142:339–346
Counter SA (1976) An electrophysiological study of sound sensitive neurons in the ‘primitive ear’ ofAcheta domesticus. J Insect Physiol 22:1–8
Daley DL, Delcomyn F (1980a) Modulation of the excitability of cockroach giant interneurons during walking. I. Simultaneous excitation and inhibition. J Comp Physiol 138:231–239
Daley DL, Delcomyn F (1980b) Modulation of the excitability of cockroach giant interneurons during walking. II. Central and peripheral components. J Comp Physiol 138:241–251
Dambach M, Rausche HG, Wendler G (1983) Proprioceptive feedback influences the calling song of the field cricket. Naturwissenschaften 70:417
Dumpert K, Gnatzy W (1977) Cricket combined mechanoreceptors and kicking response. J Comp Physiol 122:9–25
Edwards JS, Palka J (1974) The cerci and abdominal giant fibres of the house cricket,Acheta domesticus. I. Anatomy and physiology of normal adults. Proc R Soc Lond Ser B 185:83–103
Elliot JH, Koch UT (1983) Sensory feedback stabilizing reliable stridulation in the field cricketGryllus campestris L. Anim Behav 31:887–901
Elliot, JCH, Koch UT, Schäffner KH, Huber F (1982) Wing movements during stridulation are affected by mechanosensory input from wing hair plates. Naturwissenschaften 69:288
Gnatzy W, Tautz J (1980) Ultrastructure and mechanical properties of an insect mechanoreceptor: Stimulustransmitting structures and sensory apparatus of the cercal filiform hairs ofGryllus. Cell Tissue Res 213:441–463
Huber F (1983) Implications of insect neuroethology for studies on vertebrates. In: Ewert JP, Capranica RR, Ingle DJ (eds) Advances in vertebrate neuroethology. NATO ASI Series A: Life Sciences 56:91–138
Jacobs G (1983) Thesis, New York State University, Albany
Kämper G (1981) Untersuchungen zur Erzeugung, Rezeption und Verarbeitung von niederfrequentem Schall bei Grillen. Thesis, Universität zu Köln
Kämper G, Dambach M (1981) Response of the cercus-to-giant interneuron system in crickets to species-specific song. J Comp Physiol 141:311–317
Kanou M, Shimozawa T (1984) A threshold analysis of cricket cereal interneurons by an alternating air-current stimulus. J Comp Physiol A154:357–365
Levine RB, Murphey RK (1980a) Pre- and postsynaptic inhibition of identified giant interneurons in the cricket (Acheta domesticus). J Comp Physiol 135:269–282
Levine RB, Murphey RK (1980b) Loss of inhibitory synaptic input to cricket sensory interneurons as a consequence of partial deafferentation. J Neurophysiol 43:383–394
Matsumoto SG, Murphey RK (1977) The cercus-to-giant interneuron system of crickets. IV. Patterns of connectivity between receptors and the medial giant interneuron. J Comp Physiol 119:319–330
Mendenhall B, Murphey RK (1974) The morphology of cricket giant interneurons. J Neurobiol 5:565–580
Morrissey RE, Edwards JS (1981) Effects of ethanol on sensory processing in the central nervous system of an insect: the cercal-to-giant interneuron system of the house cricket. Comp Biochem Physiol 70C:159–169
Möss D (1971) Sense organs in the wing region of the field cricket (Gryllus campestris L.) and their role in the control of stridulation and wing position. Z Vergl Physiol 73:53–83
Murphey RK, Palka J, Hustert R (1977) The cercus-to-giant interneuron system of crickets. II. Response characteristics of two giant interneurons. J Comp Physiol 119:285–300
Palka J, Olberg R (1977) The cercus-to-giant interneuron system of crickets. III. Receptive field organization. J Comp Physiol 119:301–317
Ritzmann RE (1981) Motor responses to paired stimulation of giant interneurons in the cockroachPeriplaneta americana. II. The ventral giant interneurons. J Comp Physiol 143:71–80
Ritzmann RE, Pollack AJ (1981) Motor responses to paired stimulation of giant interneurons in the cockroachPeriplaneta americana. I. The dorsal giant interneurons. J Comp Physiol 143:61–70
Ritzmann RE, Tobias ML, Fourtner CR (1980) Flight activity initiated via giant interneurons of the cockroach: Evidence for bifunctional trigger interneurons. Science 210:443–445
Rozhkova GI (1980) Comparison of the constancy mechanisms in the cereal systems of crickets (Acheta domesticus andGryllus bimaculatus). J Comp Physiol 137:287–296
Schäffner KH, Koch UT (1983) Regulation of cricket stridulation by sensory input from the wings. Verh Dtsch Zool Ges 1983:199
Schildberger K (1984) Multimodal interneurons in the cricket brain: properties of identified extrinsic mushroom body cells. J Comp Physiol A 154:71–79
Schwab WE, Josephson RK (1977) Coding of acoustic information in cockroach giant fibres. J Insect Physiol 23:665–670
Steward WW (1978) Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimid tracer. Cell 14:741–759
Tautz J (1979) Reception of particle oscillation in a medium — an unorthodox sensory capacity. Naturwissenschaften 66:452–461
Thorson J, Weber T, Huber F (1982) Auditory behaviour of the cricket. II. Simplicity of calling-song recognition inGryllus, and anomalous phonotaxis at abnormal carrier frequencies. J Comp Physiol 146:361–378
Tobias M, Murphey RK (1979) The response of cereal receptors and identified interneurons in the cricket (Acheta domesticus) to airstreams. J Comp Physiol 129:51–59
Vardi N, Camhi JM (1982) Functional recovery from lesions in the escape system of the cockroach. I. Behavioural recovery. J Comp Physiol 146:291–298
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kämper, G. Abdominal ascending interneurons in crickets: responses to sound at the 30-Hz calling-song frequency. J. Comp. Physiol. 155, 507–520 (1984). https://doi.org/10.1007/BF00611915
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00611915