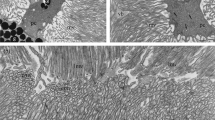
Summary
-
1.
To test if the light-evoked hyperpolarization of the rods or retinal pigment epithelium (RPE) is important for the light-evoked component of rod disc shedding, eyecups of the anuran,Xenopus, were incubated in media that have been reported to have different effects on the membrane potential of these cells.
-
2.
Hyperpolarization was induced by transferral to medium with Na+ entirely replaced by choline+, or mostly replaced by Li+. Shedding in darkness was increased 4-fold in both cases (Figs. 1, 2 and 3).
-
3.
To prevent light-evoked hyperpolarization, eyecups were transferred to medium containing 0.5 mmol/l ouabain. However, light-evoked shedding was not inhibited; instead, it was activated further (Fig. 4). Moreover, ouabain increased shedding in darkness by about 9-fold (Fig. 5).
-
4.
Ouabain likewise activated shedding without light in eyecups of the frog,Rana (Fig. 6). InRana, shedding is entirely light-evoked, so that, unlike the case represented byXenopus eyecups, there was no question that inhibition of the light-evoked component could have been masked by over-whelming stimulation of an endogenous component.
-
5.
Strophanthidin, which, in contrast to ouabain, binds reversibly to Na+-K+-ATPase, also activated shedding inXenopus eyecups kept in darkness, even when it was washed out 20 min after light onset (Fig. 7).
-
6.
These results suggest that a particular change in the membrane potential of the rod or RPE cells is not directly responsible for the occurrence of light-evoked rod disc shedding.
-
7.
In addition, the results identify pharmacological treatments that activate shedding in darkness. These treatments should provide a useful tool for future studies of rod disc shedding. It is noted that common effects of these treatments possibly include: (1) inhibition of Na+, K+-ATPase activity, with a resulting increase in ATP; (2) an increase in intracellular Ca2+; (3) alteration of melatonin levels.
Similar content being viewed by others
Abbreviations
- ROS :
-
rod outer segment
- RPE :
-
retinal pigment epithelium
- Na +,K + -ATPase :
-
Sodium, potassium-activated adenosine triphosphatase
References
Basinger SF, Hollyfield JG (1980) Control of rod shedding in the frog retina. Neurochem 1:81–92
Basinger S, Hoffman R, Matthes M (1976) Photoreceptor shedding is initiated by light in the frog retina. Science 194:1074–1076
Bastian BL, Fain GL (1982) The effects of sodium replacement on the responses of toad rods. J Physiol (Lond) 330:331–347
Besharse JC, Dunis DA (1983) Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science 219:1341–1343
Besharse JC, Hollyfield JG, Rayborn MI (1977) Turnover of rod photoreceptor outer segments. II. Membrane addition and loss in relationship to light. J Cell Biol 75:507–527
Besharse JC, Terrill RO, Dunis DA (1980) Light-evoked disc shedding by rod photoreceptors in vitro: relationship to medium bicarbonate concentration. Invest Ophthalmol Visual Sci 19:1512–1517
Besharse JC, Dunis DA, Burnside B (1982) Effects of cyclic adenosine 3′,5′-monophosphate on photoreceptor disc shedding and retinomotor movement. J Gen Physiol 79:775–790
Blaustein MP (1974) The interrelationship between sodium and calcium fluxes across cell membrane. Rev Physiol Biochem Pharmacol 70:33–74
Bok D (1982) Autoradiographic studies on the polarity of plasma membrane receptors in retinal pigment epithelium. In: Hollyfield JG (ed) The structure of the eye. Elsevier, NewYork, pp 247–256
Bortoff A (1964) Localization of slow potential responses in theNecturus retina. Vision Res 4:627–636
Brown JE, Pinto LH (1974) Ionic mechanisms for the photoreceptor potential of the retina ofBufo marinus. J Physiol (Lond) 236:575–591
Cervetto L (1973) Influence of sodium, potassium and chloride ions on the intracellular responses of turtle photoreceptors. Nature 241:401–403
Dunham PB, Hoffman JF (1971) Active cation transport and ouabain binding in high potassium and low potassium red blood cells of sheep. J Gen Physiol 58:94–116
Flannery JG, Fisher SK (1979) Light-triggered rod disc shedding inXenopus retina in vitro. Invest Ophthalmol Visual Sci 18:638–642
Frank RN, Goldsmith TH (1967) Effects of cardiac glycosides on electrical activity in the isolated retina of the frog. J Gen Physiol 50:1585–1606
Gorman ALF, Marmor MF (1974) Long-term effect of ouabain and sodium pump inhibition on a neuronal membrane. J Physiol (Lond) 242:59–60
Greenberger LM, Besharse JC (1983) Photoreceptor disc shedding in eye cups: inhibition by deletion of extracellular divalent cations. Invest Opthalmol Visual Sci 24:1456–1464
Heath AR, Basinger SF (1983) Frog rod outer segment shedding in vitro: histologic and electrophysiologic observations. Invest Ophthalmol Visual Sci 24:277–284
Hoffman JF (1966) The red cell membrane and the transport of sodium and potassium. Am J Med 41:666–680
Hoffman JF (1969) The interaction between tritiated ouabain and the Na-K pump in red blood cells. J Gen Physiol 54:3435–3505
Kimble EA, Svobody RA, Ostroy SE (1980) Oxygen consumption and ATP changes of the vertebrate photoreceptor. Exp Eye Res 31:271–288
Kouri J, Noa M, Diaz B, Niubo E (1980) Hyperpolarization of rat peritoneal macrophages phagocytosing latex particles. Nature 283:868–870
LaVail MM (1976) Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science 194:1071–1074
Leibovic KN (1983)Bufo rod responses reveal a contribution of the sodium pump. Soc Neurosci Abstr 9:164
Lipton SA, Ostroy SE, Dowling JE (1977) Electrical and adaptive properties of rod photoreceptors inBufo marinus. I. Effects of altered extracellular Ca2+ levels. J Gen Physiol 70:747–770
Masterson E, Chader GJ (1981) Pigment epithelial cells in culture: metabolic pathways required for phagocytosis. Invest Ophthalmol Visual Sci 20:1–7
Miller SS, Steinberg RH (1977a) Passive ionic properties of frog retinal pigment epithelium. J Membr Biol 36:337–372
Miller SS, Steinberg RH (1977b) Active transport of ions across frog retinal pigment epithelium. Exp Eye Res 25:235–248
Oakley B (1983) Potassium homeostasis in the toad retina. Invest Ophthalmol Visual Sci 23 (Suppl): 179
Oakley B, Flaming DG, Brown KT (1979) Effects of rod receptor potential upon retinal extracellular potassium concentration. J Gen Physiol 74:713–737
Ogino N, Matsumura M, Shirakawa H, Tsukuhara I (1983) Phagocytic activity of cultured retinal pigment epithelial cells from chick embryo; inhibition by melatonin and cyclic AMP, and its reversal by taurine and cyclic GMP. Ophthalmic Res 15:72–89
Okada Y, Tsuchiya W, Yada T, Yano J, Yawo H (1981) Phagocytic activity and hyperpolarizing responses in L-strain mouse fibroblasts. J Physiol (Lond) 313:101–119
Osborne NN (1982) Uptake, localization, and release of serotonin in the chick retina. J Physiol (Lond) 331:469–479
Parfitt A, Weller JL, Klein DC, Sakai KK, Marks BH (1975) Blockade by ouabain or elevated potassium ion concentration of the adrenergic and adenosine cyclic 3′,5′-monophosphate-induced stimulation of pineal serotonin N-acetyl-transferase activity. Mol Pharmacol 11:241–255
Sachs JR (1974) Interaction of external K, Na, and cardioactive steroids with the Na-K pump of the human red blood cell. J Gen Physiol 63:123–143
Schnetkamp PPM (1980) Ion selectivity of the cation transport system of isolated intact cattle rod outer segments. Biochim Biophys Acta 598:66–90
Steinberg RH, Miller SS (1973) Aspects of electrolyte transport in frog pigment epithelium. Exp Eye Res 16:365–372
Steinberg RH, Schmidt R, Brown KT (1970) Intracellular responses to light from cat pigment epithelium: origin of the electroretinogram c-wave. Nature 227:728–730
Stowe S (1983) Light-induced and spontaneous breakdown of the rhabdoms in a crab at dawn; depolarization versus calcium levels. J Comp Physiol 153:365–375
Thomas TN, Redburn DA (1979) 5-hydroxytryptamine — a neurotransmitter of bovine retina. Exp Eye Res 28:55–61
Tomita T (1970) Electrical activity of vertebrate photoreceptors. Q Rev Biophys 3:179–222
Torre V (1982) The contribution of the electrogenic sodium-potassium pump to the electrical activity of toad rods. J Physiol (Lond) 333:315–341
Trachtenberg MC, Packey DJ, Sweeney T (1981) In vivo functioning of the Na+, K+-activated ATPase. In: Horecker BL, Stadtman ER (eds) Current topics in cellular regulation. Academic Press, New York, vol 19, pp 159–217
Williams DS, Wilson C, Fisher SK (1983) Activation of rod outer segment shedding: the unimportance of membrane potential. Soc Neurosci Abstr 9:503
Winkler BS (1981) Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol 77:667–692
Winkler BS, Simson V, Benner J (1977) Importance of bicarbonate in retinal function. Invest Ophthalmol Visual Sci 16:766–768
Woodruff ML, Fain GL, Bastian BL (1982) Light-dependent ion influx into toad photoreceptors. J Gen Physiol 80:517–536
Young RW, Bok D (1969) Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol 42:392–403
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Williams, D.S., Wilson, C., Linberg, K. et al. Effects of low sodium, ouabain, and strophanthidin on the shedding of rod outer segment discs. J. Comp. Physiol. 155, 763–770 (1984). https://doi.org/10.1007/BF00611593
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00611593