Summary
-
1.
Anuran tongue is controlled by visual stimuli for releasing the prey-catching behavior (‘snapping’) and also by the intra-oral stimuli for eliciting the lingual reflex. To elucidate the neural mechanisms controlling tongue movements, we analyzed the neuronal pathways from the glossopharyngeal (IX) afferents to the hypoglossal (XII) tongue-muscle motoneurons.
-
2.
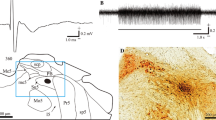
Field potentials were recorded from the bulbar dorsal surface over the fasciculus solitarius (fsol) to the electrical stimulation of the ipsilateral IX nerve. They were composed of three successive negative waves: S1, S2 and N wave. The S1 and S2 waves followed successive stimuli applied at short intervals (10 ms or less), whereas the N wave was strongly suppressed at intervals shorter than 500 ms. Furthermore, the S1 wave had lower threshold than the S2 wave.
-
3.
Orthodromic action potentials were intra-axonally recorded from IX afferent fibers in the fsol to the ipsilateral IX nerve stimuli. Two peaks found in the latency distribution histogram of these action potentials well coincided with the negative peaks of the S1 and the S2 waves of the simultaneously recorded field potentials. Therefore, the S1 and S2 waves should represent the compound action potentials of two groups of the IX afferent fibers with different conduction velocities.
-
4.
Ipsilateral IX nerve stimuli elicited excitatory postsynaptic potentials (EPSPs) in the tongue-protractor motoneurons (PMNs) and the tongue-re-tractor motoneurons (RMNs). Inhibitory postsynaptic potentials were not observed.
-
5.
The EPSPs recorded in PMNs had mean onset latencies of 6.4 ms measured from the negative peaks of the S1 wave. The EPSPs were facilitated when paired submaximal stimuli were applied at intervals shorter than 20 ms, but were suppressed at intervals longer than 30 ms. Furthermore, the EPSPs were spatially facilitated when peripherally split two bundles of the IX nerve were simultaneously stimulated.
-
6.
On the other hand, the EPSPs recorded in RMNs had shorter onset latencies, averaging 2.5 ms. In 14 of 43 RMNs, early and late EPSP components could be reliably discriminated. The thresholds for the early EPSP components were as low as those for the S1 waves, whereas for the late EPSP components the thresholds were usually higher than those for the S2 waves. The early EPSP components had mean onset latencies of 2.3 ms and followed each of the successive stimuli applied at short intervals with constant latencies and amplitudes. On the other hand, the late EPSP components appeared at 5.3 ms after the onset of the early EPSP components, and were temporally facilitated by successive stimuli at 10 ms intervals.
-
7.
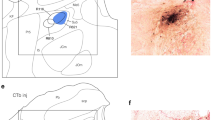
Double-labeling experiments (horseradish peroxidase and cobaltic-lysine) revealed that some of the IX afferent terminals with beaded bouton-like structures had direct contacts with the dorsal and lateral dendrites and the somata of the XII motoneurons, but not with their medial dendrites. These direct contacts only occurred in the rostral region of the dorsomedial XII nucleus, where RMNs are reported to predominate.
-
8.
Contralateral IX nerve stimuli also elicited EPSPs in both PMNs and RMNs. Latencies measured from the onset of stimuli averaged 10.6 ms and 10.8 ms for PMNs and RMNs, respectively. These EPSPs were smaller than those evoked by the ipsilateral IX nerve stimuli, and were often facilitated by successive stimuli at 10 ms intervals.
-
9.
It appears that the ipsilateral IX nerve afferents connect to PMNs polysynaptically, while they connect to RMNs by two kinds of excitatory pathways: short-latency monosynaptic pathways and longer-latency polysynaptic pathways. Furthermore, it appeared that the contralateral IX nerve afferents connect to both of PMNs and RMNs via polysynaptic excitatory pathways.
-
10.
We also demonstrated a spatial facilitation between the polysynaptic EPSPs evoked by the IX nerve stimuli and those by the stimulations of the ‘snapping’-evoking area in the optic tetum (OT). These results suggest that some common excitatory interneurons, on which the tectal descending volleys and the IX nerve afferent volleys converge, mediate polysynaptic activation of the tonguemuscle motoneurons.
Similar content being viewed by others
Abbreviations
- PMN :
-
protractor motoneuron
- RMN :
-
retractor motoneuron
- IX :
-
glossopharyngeal
- XII :
-
hypoglossal
- OT :
-
optic tectum
- fsol :
-
fasciculus solitarius
- EPSP :
-
excitatory postsynaptic potential
- IPSP :
-
inhibitory postsynaptic potential
References
Antal M (1985) The cobalt as a neuronal tracer. Acta Medica Kinki University 10:1–10
Antal M, Matsumoto N, Székely G (1986) Tectal neurons of the frog: intracellular recording and labeling with cobalt electrodes. J Comp Neurol 246:238–253
Bhattacharya CG (1967) A simple method of resolution of a distribution into Gaussian components. Biometrics 23:115–135
Biscoe TJ, Duggan AW, Lodge D (1972) The inhibition of hypoglossal motoneurones by glossopharyngeal nerve stimulation in the rat. J Physiol 226:71
Bowman JP (1968) Muscle spindles in the intrinsic and extrinsic muscles of the rhesus monkey's (Macaca mulatta) tongue. Anat Rec 161:483–488
Brookhart JM, Fadiga E (1960) Potential fields initiated during monosynaptic activation of frog motoneurones. J Physiol 150:633–655
Carew TJ (1981) Spinal cord II: reflex action. In: Kandel ER, Schwartz JH (eds) Principles of neural science. Elsevier, New York Amsterdam Oxford, pp 293–304
Carlsen RC, Mendell LM (1977) A comparison of the reflex organization of thoracic and lumbar segments in the frog spinal cord. Brain Res 124:415–426
Cooper S (1953) Muscle spindles in the intrinsic muscles of the human tongue. J Physiol 122:193–202
Cooper S (1954) Afferent impulses in the hypoglossal nerve on stretching the cat's tongue. J Physiol 126:32
Dean J (1980a) Encounters between bombardier beetles and two species of toads (Bufo americanus, B. marinus): speed of prey-capture does not determine success. J Comp Physiol 135:41–50
Dean J (1980b) Effect of thermal and chemical components of bombardier beetle chemical defense: glossopharyngeal response in two species of toads (Bufo americanus, B. marinus). J Comp Physiol 135:51–59
Duggan AW, Lodge D, Biscoe TJ (1973) The inhibition of hypoglossal motoneurones by impulses in the glossopharyngeal nerve of the rat. Exp Brain Res 17:261–270
Ewert J-P (1967) Aktivierung der Verhaltensfolge beim Beutefang der Erdkröte (Bufo bufo L.) durch elektrische Mittelhirnreizung. Z Vergl Physiol 54:455–481
Ewert J-P (1984) Tectal mechanisms that underly prey-catching and avoidance behaviors in toads. In: Vanegas H (ed) Comparative neurology of the optic tectum. Plenum Press, New York, pp 247–416
Gans C, Gorniak GC (1982a) How does the toad flip its tongue? Test of two hypotheses. Science 216:1335–1337
Gans C, Gorniak GC (1982b) Functional morphology of lingual protrusion in marine toads (Bufo marinus). Am J Anat 163:195–222
Gaupp E (1896–1904) A. Ecker's and R. Wiedersheim's Anatomie des Frosches, vol I–III. Vieweg, Braunschweig
Ghez C (1981) Introduction to the motor systems. In: Kandel ER, Schwartz JH (eds) Principles of neural science. Elsevier, New York Amsterdam Oxford, pp 271–283
Görcs T, Antal M, Oláh E, Székely G (1979) An improved cobalt labeling technique with complex compounds. Acta Biol Acad Sci Hung 30:79–86
Grobstein P, Comer C, Kostyk SK (1983) Frog prey capture behavior: between sensory maps and directed motor output. In: Ewert J-P, Capranica RR, Ingle DJ (eds) Advances in vertebrate neuroethology. Plenum Press, London New York, pp 331–347
Grüsser OJ, Grüsser-Cornehls U (1976) Physiology of the anuran visual system. In: Llinás R, Precht W (eds) Frog neurobiology. Springer, Berlin Heidelberg New York, pp 297–385
Hanamori T, Ishiko N (1981) Conduction velocity of the IXth nerve fibers innervating taste organs in the rostral and caudal tongue region in bullfrog. Chem Senses 6:175–187
Hanamori T, Ishiko N (1983a) Surface and intramedullary potentials evoked by stimulation of the glossopharyngeal nerve in frogs. Brain Res 260:51–60
Hanamori T, Ishiko N (1983b) Intraganglionic distribution of the primary afferent neurons in the frog glossopharyngeal nerve and its transganglionic projection to the rhombencephalon studied by HRP method. Brain Res 260:191–199
Ingle DJ (1983) Brain mechanisms of visual localization by frogs and toads. In: Ewert J-P, Capranica RR, Ingle DJ (eds) Advances in vertebrate neuroethology. Plenum Press, London New York, pp 177–226
Jankovska E, Lundberg A (1981) Interneurons in the spinal cord. Trends Neurosci 4:230–233
Kozai H (1974) Reflex discharge of hypoglossal nerve of frogs. J Kyushu Dent Sci 29:210–221
Kumai T (1981a) Reflex response of the hypoglossal nerve induced by gustatory stimulation of the frog tongue. Brain Res 208:432–435
Kumai T (1981b) Reflex response of the hypoglossal nerve induced by chemical stimulation of the tongue and electrical stimulation of the glossopharyngeal nerve in the frog. Jpn J Physiol 31:625–637
Lázár G (1969) Efferent pathways of the optic tectum in the frog. Acta Biol Acad Sci Hung 20:171–183
Lázár G, Tóth P, Csank G, Kicliter E (1983) Morphology and location of tectal projection neurons in frogs: a study with HRP and cobalt-filling. J Comp Neurol 215:108–120
Low ME (1954) Lingual proprioception in pig, dog and cat. Nature 174:1107–1108
Lowe AA (1981) The neural regulation of the tongue movements. Progr Neurobiol 15:295–344
Lundberg A (1970) The excitatory control of Ia inhibitory pathway. In: Anderson P, Jansen JKS (eds) Excitatory synaptic mechanisms. Universitetsforlaget, Oslo, pp 333–340
Matesz C, Székely G (1978) The motor column and sensory projections of the branchial cranial nerves in the frog. J Comp Neurol 178:157–176
Matsumoto N (1984) Cobaltic lysine as a neuronal tracer. Seitai no kagaku 35:304–308
Matsushima T, Satou M, Ueda K (1984a) Synaptic mechanism of glossopharyngeal-hypoglossal reflex in the toad. J Physiol Soc Jpn 46:378
Matsushima T, Satou M, Ueda K (1984b) Toad's snapping pathway: relationship to the excitatory pathways from glossopharyngeal nerve to tongue-muscle motoneurons. Zool Sci 1:881
Matsushima T, Satou M, Ueda K (1985a) An electromyographic analysis of electrically-evoked prey-catching behavior by means of stimuli applied to the optic tectum in the Japanese toad. Neurosci Res 3:154–161
Matsushima T, Satou M, Ueda K (1985b) Toad's snapping pathway: a search for the premotor interneurons. Zool Sci 2:874
Matsushima T, Satou M, Ueda K (1986) Glossopharyngeal and tectal influences on tongue-muscle motoneurons in the Japanese toad. Brain Res 365:198–203
Matsushima T, Satou M, Ueda K (1987) Direct contacts between glossopharyngeal afferent terminals and hypoglossal motoneurons revealed by double labeling with cobaltic-lysine and horseradish peroxidase in the Japanese toad. Neurosci Lett 80:241–245
Morimoto T, Kawamura Y (1971) Discharge patterns of hypoglossal afferents in a cat. Brain Res 35:539–542
Morimoto T, Takata M, Kawamura Y (1968) Effect of lingual nerve stimulation on hypoglossal motoneurons. Exp Neurol 22:174–190
Nakachi T, Ishiko N (1986) Gustatory signal processing in the glossopharyngeo-hypoglossal reflex arc of the frog. Jpn J Physiol 36:189–208
Nakahara S, Liou P, Izumi E, Ohmagari T, Momose Y (1969) The efferent impulses of the hypoglossal nerve by a variety of stimulations in the tongue of frog. J Kyushu Dent Sci 22:345–352
Niewenhuys R, Opdam P (1976) Structure of the brain stem. In: Llinás R, Precht W (eds) Frog neurobiology. Springer, Berlin Heidelberg New York, pp 811–855
Oka Y, Satou M, Ueda K (1987a) Morphology and distribution of the accessory nerve (nXI) in the Japanese toad: a cobaltic lysine study. Brain Res 400:383–388
Oka Y, Takeuchi H, Satou M, Ueda K (1987b) Morphology and distribution of the preganglionic parasympathetic neurons of the facial, glossopharyngeal and vagus nerves in the Japanese toad: a cobaltic lysine study. Brain Res 400:389–395
Oka Y, Takeuchi H, Satou M, Ueda K (1987c) A cobaltic lysine study of the morphology and distribution of the cranial nerve efferent neurons (motoneurons and preganglionic parasympathetic neurons) and rostral spinal motoneurons in the Japanese toad. J Comp Neurol 259:400–423
Porter R (1967) Cortical actions on hypoglossal motoneurons in cats: a proposed role for a common internuncial cell. J Physiol 193:293–308
Potter HD (1965) Mesencephalic auditory region of the bullfrog. J Neurophysiol 28:1132–1154
Roth G (1987) Visual behavior in salamander. Springer, Berlin Heidelberg New York, pp 269–275
Rubinson K (1968) Projections of the tectum opticum of the frog. Brain Behav Evol 1:529–561
Sato M (1976) Physiology of the gustatory system. In: Llinás R, Precht W (eds) Frog neurobiology. Springer, Berlin Heidelberg New York, pp 576–587
Satou M, Matsushima T, Ueda K (1983 a) Prey-catching behavior in the Japanese toad: analyses of the excitatory pathway from the optic tectum to the tongue-muscle-controlling motoneurons. Proc 5th Annu Meet Jpn Soc Gen Comp Physiol, p79
Satou M, Mori K, Tazawa Y, Takagi SF (1983b) Neuronal pathways for activation of inhibitory interneurons in pyriform cortex of the rabbit. J Neurophysiol 50:74–88
Satou M, Matsushima T, Ueda K (1984) Neuronal pathways from the tectal ‘snapping-evoking area’ to the tongue-muscle-controlling motoneurons in the Japanese toad: evidence of the intervention of excitatory interneurons. Zool Sci 1:829–832
Satou M, Matsushima T, Takeuchi H, Ueda K (1985) Tongue-muscle-controlling motoneurons in the Japanese toad: topography, morphology and neuronal pathways from the ‘snapping-evoking area’ in the optic tectum. J Comp Physiol A 157:717–737
Satou M, Takeuchi H, Ueda K (1985) Effects of electrical stimulation of dorsal thalamic area on activities of tongue-muscle-controlling motoneurons in the Japanese toad. Zool Sci 2:873
Shapovalov AI, Shiriaev BI (1980) Dual mode of junctional transmission at synapses between single primary fibres and motoneurones in the amphibian. J Physiol 306:1–15
Simpson JI (1976) Functional synaptology of the spinal cord. In: Llinás R, Precht W (eds) Frog neurobiology. Springer, Berlin Heidelberg New York, pp 728–749
Stuesse SL, Cruce WLR, Powell K (1983) Afferent and efferent components of the hypoglossal nerve in the grass frogRana pipiens. J Comp Neurol 217:432–439
Stuesse SL, Cruce WLR, Powell KS (1984) Organization within the cranial IX-X complex in ranid frogs: a horseradish peroxidase transport study. J Comp Neurol 222:358–365
Suzuki N (1966) Taste mechanism in the frog's palate. Zool Mag 75:239–246
Székely G, Levai G, Matesz K (1983) Primary afferent terminals in the nucleus of the solitary tract of the frog: an electron microscopic study. Exp Brain Res 53:109–117
Takei K, Oha Y, Satou M, Ueda K (1984) Localization of motoneurons involved in the prey-catching behavior in the Japanese toad. Zool Sci 1:881
Takei K, Oka Y, Satou M, Ueda K (1987) Distribution of motoneurons involved in the prey-catching behavior in the Japanese toad,Bufo japonicus. Brain Res 410:395–400
Takeuchi H, Satou M, Ueda K (1986) EMG analysis of head muscles during naturally-occurring and electrically-evoked snapping, rejecting and avoiding behavior in the Japanese toad. Zool Sci 3:992
Tóth P, Szábo T (1986) Simultaneous labelling of two different central nervous system pathways with horseradish peroxidase and cobalt inGnathonemus petersii andRana esculenta. Neurosci Lett 64:350–354
Weerasuriya A (1983) Snapping in toads: some aspects of sensorimotor interfacing and motor pattern generation. In: Ewert J-P, Capranica RR, Ingle DJ (eds) Advances in vertebrate neuroethology. Plenum Press, New York London, pp 613–627
Weerasuriya A, Ewert J-P (1981) Prey-selective neurons in the toad's optic tectum and sensorimotor interfacing: HRP studies and recording experiments. J Comp Physiol 144:429–434
Yamashita M (1986) Monosynaptic connexions of low threshold muscle afferents with hindlimb motoneurones in the turtle spinal cord. Exp Brain Res 63:519–529
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matsushima, T., Satou, M. & Ueda, K. Neuronal pathways for the lingual reflex in the Japanese toad. J. Comp. Physiol. 164, 173–193 (1988). https://doi.org/10.1007/BF00603949
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00603949