Abstract
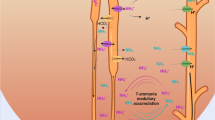
γ-Glutamyltransferase (γ-GT) is located in the brushborder membrane of the proximal tubule where the catalytic site of the enzyme faces the lumen. The (phosphate-independent) glutaminase activity of γ-GT in vitro is activated by hippurate. In order to investigate glutamine deamidation in the tubule lumen in vivo,14C-l-glutaminecontaining solutions were continuously microperfused through sections of the proximal convoluted tubule in vivo and in situ.d-aspartate andl-phenylalanine (10 mmol/l, each) were added to the perfusate in order keep the reabsorption ofl-glutamine as such low and to block reabsorption of any glutamate possibly formed, respectively. Intraluminal formation of glutamate from glutamine in the absence of hippurate is small. In presence of 10 mmol/l hippurate, 5%–70% of the recovered14C-activity was14C-glutamate at an initial14C-l-glutamine concentration of 1 mmol/l. The respective absolute rate (±SEM) of glutamate formation, i.e., 36±5 pmol · s−1 · m−1, was increased 1.4-fold at an initiall-glutamine concentration of 3 mmol/l, but dropped to one third at initially 0.3 mmol/l. A rough estimate of the apparent kinetic constants resulted in aK m of 0.58 (0.19–0.97) mmol/l and aV max of 56 (40–93) pmol · s−1 · m−1. Deamidation of glutamine occurred also in the absence ofl-phenylalanine. Acivicin (AT 125), a γ-GT inhibitor, completely blocked glutamate formation. Endogenous hippurate concentrations determined by free flow micropuncture and HPLC were 0.16 mmol/l in the late proximal convulation, 0.6 mmol/l in the early distal convolution, and 4.9 mmol/l in the final urine. In conclusion, glutamine deamidation, i.e. ammoniagenesis, can take place in the lumen of the proximal tubule under favourable conditions. It can be estimated that this luminal ammoniagenesis makes up about 5%–10% of the normal renal ammonium excretion in these rats at endogenous proximal hippurate levels.
Similar content being viewed by others
References
Albert Z, Orlowski M, Szewczuk A (1961) Histochemical demonstration of γ-glutamyl transpeptidase. Nature 191:767–768
Astier A, Deutsch AM (1980) High-performance liquid chromatographic determination of hippuric acid in human urine. Preliminary results for normal urine levels. J Chromatogr 182:88–93
Binkley F (1951) Metabolism of glutathione. Nature 167:888–889
Borsook H, Dubnoff J (1940) The biological synthesis of hippuric acid in vitro. J Biol Chem 132:307–324
v. Bunge G, Schmiedeberg O (1876–77) Arch Exp Path Pharmak 6:233 (quoted from Borsook, see above)
Curthoys NP (1983) Role of γ-glutamyltranspeptidase in the renal metabolism of glutathione. Min Electrolyte Metab 9:236–245
Curthoys NP, Godfrey SS (1976) Properties of rat kidney glutaminase enzymes and their role in renal ammoniagenesis. In: Schmidt U, Dubach UC (eds) Renal metabolism in relation to renal function. Huber, Bern Stuttgart Vienna, pp 346–356
Curthoys NP, Hughey RP (1979) Characterization and physiological function of rat renal γ-glutamyltranspeptidase. Enzyme 24:383–403
Curthoys NP, Kuhlenschmidt T (1975) Phosphate-independent glutaminase from rat kidney. Partial purification and identity with γ-glutamyltranspeptidase. J Biol Chem 250:2099–2105
Curthoys NP, Lowry OH (1973) Glutamate and glutamine distribution in the rat nephron in acidosis and alkalosis. Am J Physiol 224:884–889
Curthoys NP, Lowry OH (1973) The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. J Biol Chem 248:162–168
Curthoys NP, Shapiro RA, Haser WG (1984) Enzymes of renal glutamine metabolism. In: Häussinger D, Sies H (eds) Glutamine metabolism in mammalian tissues. Springer, Berlin Heidelberg New York Tokyo, pp 16–31
Dass PD, Welbourne TC (1982) Effect of AT-125 on in situ renal γ-glutamyltransferase activity. FEBS Lett 144:21–24
Elce JS, Broxmeyer B (1976) γ-Glutamyltransferase of rat kidney. Simultaneous assay of the hydrolysis and transfer reactions with (glutamate-14C)-glutathione. Biochem J 153:223–232
Endou H, Shimada H, Koseki C, Yokokura Y, Sakai F (1981) Distribution and possible functions of γ-glutamyl-transpeptidase in the kidney. Jpn J Nephrol 23:981–988
Fonteles MC, Pillion DJ, Jeske AH, Leibach FH (1976) Extraction of glutathione by the isolated perfused rabbit kidney. J Surg Res 21:169
Glenner GG, Folk JE (1961) Glutamyl peptidase in rat and guinea pig kidney slices. Nature 192:338–340
Glossmann H, Neville Jr DM (1972) γ-Glutamyltransferase in kidney brush border membranes. FEBS Lett 19:340–344
Griffith OW (1981) The role of glutathione turnover in the apparent renal secretion of cystine. J Biol Chem 256:12253–12268
Griffith OW, Meister A (1980) Excretion of cysteine and γ-glutamylcysteine moieties in human and experimental animal γ-glutamyl transpeptidase deficiency. Proc Natl Acad Sci USA 77:3384–3387
Heinle H, Wendel A (1977) The activity of the key enzymes of the γ-glutamyl cycle in microdissected segments of the rat nephron. FEBS Lett 73:220–224
Hendrix BM, Sanders JP (1923) The effect of injections of sodium phosphates and sodium hippurate upon the excretion of acid and ammonia by the kidney. J Biol Chem 58:503–513
Horiuchi S, Inoue M, Morino Y (1978) γ-Glutamyltranspeptidase: sidedness of its active site on renal brushborder membrane. Eur J Biochem 87:429–437
Hsu BYL, Foreman JW, Corcoran SM, Ginkinger K, Segal S (1984) Absence of a role of gamma-glutamyl transpeptidase in the transport of amino acids by rat renal brushborder membrane vesicles. J Membr Biol 80:167–173
Katunuma N, Tomino I, Nishino H (1966) Glutaminase isozymes in rat kidney. Biochem Biophys Res Commun 22:321–328
Kugler P (1981) Localization of aminopeptidase A (angiotensinase A) in the rat and mouse kidney. Histochemistry 72:269–278
Kuhlenschmidt T, Curthoys NP (1975) Subcellular localization of rat kidney phosphate independent glutaminase. Arch Biochem Biophys 167:519–524
Lemieux G, Baverel G, Vinay P, Wadoux P (1976) Glutamine synthetase and glutamyltransferase in the kidney of man, dog and rat. Am J Physiol 231:1068–1073
Marathe GV, Nash B, Haschemeyer RH, Tate SS (1979) Ultrastructural localization of γ-glutamyltranspetidase in rat kidney and jejunum. FEBS Lett 107:436–440
McFarlane-Anderson N, Alleyne GAO (1977) The effect of metabolic acidosis on γ-glutamyltranspeptidase activity in the rat kidney. FEBS Lett 79:51–53
Nonoguchi H, Uchida S, Shiigai T, Endou H (1985) Effect of chronic metabolic acidosis on ammonia production froml-glutamine in microdissected rat nephron segments. Pflügers Arch 403:229–235
O'Donovan DJ (1980) Glutamine metabolism in carbon tetrachloride-treated acidotic rats. Int J Biochem 12:85–87
Orlowski M, Meister A (1970) The γ-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci USA 67:1248
Pfaller W, Gstraunthaler G, Kotanko P, Wolf H, Curthoys NP (1984) Immunocytochemical localization of gamma-glutamyltransferase on isolated renal cortical tubular fragments. Histochemistry 80:289–293
Reed DJ, Ellis WW, Meck RA (1980) The inhibition of γ-glutamyl transpeptidase and glutathione metabolism of isolated rat kidney cells byl-(αS,5S)-α-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid (AT-125; NSC-163501). Biochem Biophys Res Com 94:1273–1277
Revel JP, Ball EG (1958) The reaction of glutathione with amino acids and related compounds as catalyzed by γ-glutamyl transpeptidase. J Biol Chem 234:577–582
Shapiro A, Curthoys NP (1981) Differential effect of AT-125 on rat renal glutaminase activities. FEBS Lett 133:131–134
Schulman JD, Goodman SI, Mace JW, Patrick AD, Tietze F, Butler EJ (1975) Glutathionuria: inborn error of metabolism due to tissue deficiency of γ-glutamyl transpeptidase. Biochem Biophys Res Com 65:68–74
Silbernagl S (1976) Renal tubular reabsorption of amino acids: specificity of the different transport systems studied by continuous microperfusion. In: Silbernagl S, Lang F, Greger R (eds) Amino acid transport and uric acid transport. Thieme, Stuttgart, pp 77–85
Silbernagl S (1977) Tubular handling of γ-glutamyl peptides and of disaccharides. A microperfusion study in rat kidney. Pflügers Arch 368:R13
Silbernagl S (1978) Renal tubular reabsorption of amino acids and peptides. In: Vogel HG, Ullrich KJ (eds) New aspects on renal function. Excerpta Medica, Amsterdam, pp 54–57
Silbernagl S (1980) Tubular reabsorption ofl-glutamine studied by free flow micropuncture and microperfusion of rat kidney. Int J Biochem 12:9–16
Silbernagl S (1981) Renal transport of amino acids and oligopeptides. In: Greger R, Lang F, Silbernagl S (eds) Renal transport of organic substances. Springer, Berlin Heidelberg New York, pp 93–117
Silbernagl S (1983) Kinetics and localization of tubular resorption of “acidic” amino acids. A microperfusion and free flow micropuncture study in rat kidney. Pflügers Arch 396:218–224
Silbernagl S (1985) Amino acids and oligopeptides. In: Seldin DW, Giebisch G (eds) The kidney: physiology and pathophysiology. Raven Press, New York, pp 1677–1707
Silbernagl S (1985) Ammoniagenesis in the lumen of the proximal tubule or not? Contrib nephrol 47:157–164
Silbernagl S, Curthoys NP, Shapiro R (1984) Are γ-glutamyl dipeptides reabsorbed as such? A microperfusion study in rat kidney. Pflügers Arch 402:R4
Silbernagl S, Curthoys NP, Shapiro R (1982) The role of renal tubular brushborder enzymes in metabolism and absorption of glutathione and cysteinyl-glycine. In: Morel F (ed) Biochemistry of kidney functions. Elsevier, Amsterdam, pp 429–437
Silbernagl S, Völkl H (1978) The role of brushborder enzymes in renal tubular transport of peptides, disaccharides and amino acids. In: Guder WG, Schmidt U (eds) Current problems in clinical biochemistry. Huber, Bern, pp 59–65
Silbernagl S, Völkl H (1983) Molecular specificity of the tubular resorption of “acidic” amino acids. A continuous microperfusion study in rat kidney in vivo. Pflügers Arch 396:225–230
Silbernagl S, Wendel A, Pfaller W, Heinle H (1978) Topology and function of renal γ-glutamyltranspeptidase (E.C. 2.3.2.2.). In: Sies H, Wendel A (eds) Functions of glutathione in liver and kidney. Springer, Berlin, pp 60–69
Smith HW, Finkelstein N, Aliminosa L, Crawford B, Graber M (1945) The renal clearances of substituted hippuric acid derivatives and other aromatic acids in dog and man. J Clin Invest 24:388
Sonnenberg H, Deetjen P (1964) Methode zur Durchströmung einzelner Nephronabschnitte. Pflügers Arch Ges Physiol 278:669–674
Tate SS, Meister A (1974) Interaction of γ-glutamyl transpeptidase with amino acids, dipeptides, and derivatives and analogs of glutathione. J Biol Chem 249:7593–7602
Tate SS, Meister A (1974) Stimulation of the hydrolytic activity and decrease of the transpeptidase activity of γ-glutamyl transpeptidase by maleate: identity of rat kidney maleate-stimulated glutaminase and γ-glutamyl transpeptidase. Proc Natl Acad Sci USA 71:3329–3334
Tate SS, Meister A (1975) Identity of maleate-stimulated glutaminase with γ-glutamyl transpeptidase in rat kidney. J Biol Chem 250:4619–4627
Tate SS, Meister A (1977) Affinity labeling of γ-glutamyl-transpeptidase and location of the γ-glutamyl binding site on the light subunit. Proc Natl Acad Sci USA 74:931–935
Thompson GA, Meister A (1979) Modulation of the hydrolysis, transfer and glutaminase activities of γ-glutamyl transpeptidase by maleate bound at the cysteinylglycine binding site of the enzyme. J Biol Chem 254:2956–2960
Thompson GA, Meister A (1980) Modulation of γ-glutamyl transpeptidase activities by hippurate and related compounds. J Biol Chem 225:2109–2113
Tsao B, Curthoys NP (1980) The absolute asymmetry of orientation of γ-glutamyltranspeptidase and aminopeptidase in the external surface of the rat renal brushborder membrane. J Biol Chem 255:7708–7711
Völkl H, Silbernagl S (1980) Molecular specificity of tubular reabsorption ofl-proline. A microperfusion study in rat kidney. Pflügers Arch 387:253–259
Völkl H, Silbernagl S, Deetjen P (1979) Kinetics ofl-proline reabsorption studied by continuous microperfusion. Pflügers Arch 382:115–121
Welbourne TC (1975) Mechanism of renal ammonia production adaptation to chronic acidosis. Med Clin North Am 59:629–647
Welbourne TC, Dass PD (1981) Mechanism of the acidosis induced adaptation in renal γ-glutamyltransferase. Life Sci 28:1219–1224
Welbourne TC, Dass PD (1981) Role of hippurate in acidosis induced adaptation in renal γ-glutamyltransferase. Life Sci 29:253–258
Wendel A, Hahn R, Guder WG (1976) On the role of γ-glutamyltransferase in renal tubular amino acid reabsorption. In: Schmidt U, Dubach UC (eds) Renal metabolism in relation to renal function. Huber, Bern, pp 426–436
Author information
Authors and Affiliations
Additional information
With the technical assistance of Katharina Völker
Rights and permissions
About this article
Cite this article
Silbernagl, S. Ammoniagenesis catalyzed by hippurate-activated γ-glutamyltransferase in the lumen of the proximal tubule. Pflugers Arch. 407 (Suppl 2), S72–S79 (1986). https://doi.org/10.1007/BF00584933
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00584933