Abstract
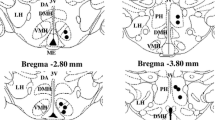
In conscious guinea-pigs the effects of electrolytic lesions of the nucleus raphe magnus (NRM) and of adjacent areas in the lower brainstem on cold- and heat-defence were studied. Changes in core, temperature, heat production and heat loss effectors as well as their threshold temperatures were compared in the same animals before and after lesioning. As an additional index of heat loss, ear skin temperature and a derived parameter — vasomotor index — were also measured. Three days after NRM lesion the fall in core temperature evoked by an exposure to 14–15°C was smaller than before lesion, furthermore the body temperature threshold for shivering increased. Cold-induced heat production was also higher following NRM lesions. Lesions outside the NRM or sham-operation did not influence colddefence. After NRM lesion heat-defence was also improved; the rise in core temperature elicited, by an exposure to 36–37°C was smaller and the body temperature threshold for car skin vasoconstriction during recovery from hyperthermia decreased. No change in respiratory evaporative heat loss could be observed after NRM lesion. Lesions outside the NRM or sham-operation did not influence, heat-defence. An attempt has been made to explain the observed improvements in cold- and heat-defence by discussing relevant data on mechanisms in central temperature control.
Similar content being viewed by others
References
Basbaum AI, Fields HL (1984) Endogenous pain control system: Brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci 7:309–338
Bobillier P, Seguin S, Petitjean F, Salvert D, Touret M, Jouvet M (1976) The raphe nuclei of the cat brain stem: a topographical atlas of their efferent projections as revealed by autoradiography. Brain Res 113:449–486
Brück K, Hinckel P (1980) Thermoregulatory noradrenergic and serotonergic pathways to hypothalamic units. J Physiol 304:193–202
Brück K, Hinckel P (1982) Thermoafferent systems and their adaptive modifications. Pharmacol Ther 17:357–381
Brück K, Hinckel P (1984) Thermal afferents to the hypothalamus and thermal adaptation. J Therm Biol 9:7–10
Brück K, Wünnenberg, W, Gallmeier H, Ziehm B (1970) Shift of threshold temperature for shivering and heat polypnea as a mode of thermal adaptation. Pflügers Arch 321:159–172
Dickenson AH (1977) Specific responses of rat raphe neurones to skin temperature. J Physiol 273:277–293
Dubner R, Bennett GJ (1983) Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci 6:381–418
Grant RT (1963) Vasodilatation and body warming in the rat. J Physiol 167:311–317
Hinckel P, Cristante L, Brück K (1983) Inhibitory effects of the lower brain stem on shivering. J Therm Biol 8:129–131
Lumb BM, Wolstencroft JH (1985) Electrophysiological studies of a rostral projection from the nucleus raphe magnus to the hypothalamus in the rat and cat. Brain Res 327:336–339
Matsumura K, Nakayama T, Ishikawa Y (1984) Effects of median raphe electrical stimulation on the preoptic thermosensitive neurons in rats. In: Hales JRS (ed) Thermal physiology Raven Press, New York, pp 87–90
Necker R, Wegner L (1981) Effects of nucleus raphe lesions and p-CPA on temperature, regulation and day-night rhythm of body temperature in pigeons. In: Szelényi Z, Székely M (eds) Contributions to thermal physiology. Pergamon, Oxford, Akadémiai Kiadó, Budapest, pp 29–31
Parent A, Poitras D, Dubé L (1984) Comparative anatomy of central monoaminergic systems. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy, vol 3, Classical transmitters and transmitter receptors in the CNS, part II. Elsevier, Amsterdam, New York, Oxford, pp 409–439
Rössner W (1965) Stereotaktischer Hirnatlas vom Meerschweinchen. Pallas, München
Steinbusch HWM (1984) Serotonin-immunoreactive neurons and their projections in the CNS. In: Björklund A, Hökfelt T, Kuhar MJ (eds) Handbook of chemical neuroanatomy, vol 3, Classical transmitters and transmitter receptors in the CNS, part II. Elsevier, Amsterdam, New York, Oxford, pp 68–125
Szelényi Z, Hinckel P (1984) Improvement of cold- and heat-defence following electrolytic lesions of raphe nuclei in the guinea-pig. Pflügers Arch 402:R39
Szelényi Z, Zeisberger E, Brück K (1977) A hypothalamic alpha-adrenergic mechanism mediating the thermogenic response to electrical stimulation of the lower brain-stem in the guinea-pig. Pflügers Arch 370:19–23
Taylor DCM (1982) The effects of nucleus raphe magnus lesions on an ascending thermal pathway in the rat. J Physiol 326:309–318
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Szelényi, Z., Hinckel, P. Changes in cold- and heat-defence following electrolytic lesions of raphe nuclei in the guinea-pig. Pflugers Arch. 409, 175–181 (1987). https://doi.org/10.1007/BF00584768
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00584768