Abstract
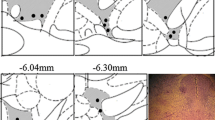
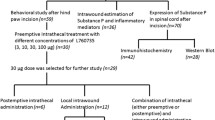
Activation of the dorsal columns is relayed to supraspinal centers, involved in pain modulation, probably via the descending fibers in the dorsolateral funiculi (DLF). The present study examines the role of the DLF in the attenuation of pain-related signs by spinal cord stimulation (SCS). Several groups of rats were subjected to nerve injury and to chronic bilateral DLF lesions at C5–7 level. In each animal, two sets of miniature electrodes were implanted, a caudal system placed in the dorsal epidural space at low thoracic level and another implanted over the dorsal column nuclei, rostral to the lesions. Stimulation (50 Hz, 0.2 ms; 70 % of motor threshold) was applied for 5 min via either of the electrodes. Behavioral tests were used to assess the effects of SCS on the nerve injury-induced mechanical and cold hypersensitivity and heat hyperalgesia. Prior to application of SCS, antagonists to either of GABAA or B, 5-HT1 or 1–2 or α/β-adrenergic receptors were injected i.p. Both stimulations produced comparable decreases (80–90 % of the control) of neuropathic manifestations in rats with intact spinal cords. DLF lesions attenuated the effects of both types of stimulation by about 50 %. Pretreatment with receptor antagonists differentially counteracted the effects of rostral and caudal stimulation; the inhibition with rostral stimulation generally being more prominently influenced. These results provide further support to the notion of important involvement of brainstem pain modulating centers in the effects of SCS. A major component of the inhibitory spinal–supraspinal–spinal loop is mediated by fibers running in the DLF.







Similar content being viewed by others
Abbreviations
- SCS:
-
Spinal cord stimulation
- DLF:
-
Dorsolateral funiculi
- DCNS:
-
Dorsal column nuclei stimulation
- SNI:
-
Spared nerve injury
- MT:
-
Motor threshold
- VF:
-
von Frey
- PWD:
-
Paw withdrawal duration
- PWL:
-
Paw withdrawal latency
- RVM:
-
Rostral ventromedial medulla
- VFL:
-
Ventrolateral funiculus
References
Akada Y, Mori R, Kato Y, Yamasaki F, Mochizuki H (2005) Analgesic properties of the novel compound M43068 in rat models of acute and neuropathic pain. Eur J Pharmacol 523:46–53
Barchini J, Tchachaghian S, Shamaa F, Jabbur SJ, Meyerson BA, Song Z, Linderoth B, Saadé NE (2012) Spinal segmental and supraspinal mechanisms underlying the pain-relieving effects of spinal cord stimulation: an experimental study in a rat model of neuropathy. Neuroscience 215:196–208
Basbaum AI, Clanton CH, Fields HL (1976) Opiate and stimulus-produced analgesia: functional anatomy of a medullospinal pathway. Proc Natl Acad Sci USA 73:4685–4688
Basbaum AI, Clanton CH, Fields HL (1978) Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J Comp Neurol 178:209–224
Burgess SE, Gardell LR, Ossipov MH, Malan TP Jr, Vanderah TW, Lai J, Porreca F (2002) Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci 22:5129–5136
Choi Y, Yoon YW, Na HS, Kim SH, Chung JM (1994) Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 59:369–376
Coimbra NC, Castro-Souza C, Segato EN, Nora JEP, Herrero CFPS, Tedeschi-Filho W, Garcia-Cairasco N (2001) Post-ictal analgesia: involvement of opioid, serotoninergic and cholinergic mechanisms. Brain Res 888:314–320
Condes-Lara M (1998) Different direct pathways of locus coeruleus to medial prefrontal cortex and centrolateral thalamic nucleus: electrical stimulation effects on the evoked responses to nociceptive peripheral stimulation. Eur J Pain 2:15–23
Cui JG, Linderoth B, Meyerson BA (1996) Effects of spinal cord stimulation on touch-evoked allodynia involve GABAergic mechanisms. An experimental study in the.mononeuropathic rat. Pain 66:287–295
Cui JG, Meyerson BA, Sollevi A, Linderoth B (1998) Effect of spinal cord stimulation on tactile hypersensitivity in mononeuropathic rats is potentiated by simultaneous GABA(B) and adenosine receptor activation. Neurosci Lett 247:183–186
Decosterd I, Woolf CJ (2000) Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87:149–158
El-Khoury C, Hawwa N, Baliki M, Atweh SF, Jabbur SJ, Saadé NE (2002) Attenuation of neuropathic pain by segmental and supraspinal activation of the dorsal column system in awake rats. Neuroscience 112:541–553
Gilbert AK, Franklin KBJ (2001) GABAergic modulation of descending inhibitory systems from the rostral ventromedial medulla (RVM). Dose-response analysis of nociception and neurological deficits. Pain 90:25–36
Guan Y (2012) Spinal cord stimulation: neurophysiological and neurochemical mechanisms of action. Curr Pain Headache Rep 16:217–225
Heinricher MM, Tortorici V (1994) Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism in nociceptive modulation. Neuroscience 63:533–546
Janssen SP, Gerard S, Raijmakers ME, Truin M, Van Kleef M, Joosten EA (2012) Decreased intracellular GABA levels contribute to spinal cord stimulation-induced analgesia in rats suffering from painful peripheral neuropathy: the role of KCC2 and GABAA receptor-mediated inhibition. Neurochem Int 60:21–30
Jones SL, Gebhart GF (1987) Spinal pathways mediating tonic, coerulospinal and raphe-spinal descending inhibition in the rat. J Neurophysiol 58:138–159
Koyama N, Hanai F, Yokota T (1998) Does intravenous administration of GABAA receptor antagonists induce both descending antinociception and touch-evoked allodynia? Pain 76:327–336
Linderoth B, Meyerson BA (2013) Spinal cord and brain stimulation. In: McMahon SB, Koltzenburg M, Tracey I, Turk DC (eds) Wall and Melzack´s textbook of pain. Elsevier/Saunders, Philadelphia, pp 570–591
Lovick TA, West DC, Wolstencroft JH (1978) Bulbar raphe neurons with projections to the spinal trigeminal nucleus and the lumbar cord in the cat. J Physiol London 277:61–62
Mantovani M, Kaster MP, Pertile R, Calixto JB, Rodrigues AL, Santos ARS (2006) Mechanisms involved in the antinociception caused by melatonin in mice. J Pineal Res 41:382–389
Melzack R, Wall PD (1965) Pain mechanisms: a new theory. Science 150:971–979
Meyerson BA, Linderoth B (2006) Mode of action of spinal cord stimulation in neuropathic pain. J Pain Symptom Manage 31:S6–S12
Meyerson BA, Herregodts P, Linderoth B, Ren B (1994) An experimental animal model of spinal cord stimulation for pain. Stereotact Funct Neurosurg 62:256–262
Millan MJ (2002) Descending control of pain. Prog Neurobiol 66:355–474
Mokha SS, McMillan JA, Iggo A (1986) Pathways mediating descending control of spinal nociceptive transmission from nuclei locus coeruleus (LC) and raphe magnus (NMR) in the cat. Exp Brain Res 61:597–606
Moreau JL, Fields HL (1986) Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res 397:37–46
Nashold BS, Somjen G, Friedman H (1972) Paresthesias and EEG potentials evoked by stimulation of the dorsal funiculi in man. Exp Neurol 36:273–287
Nyquist JK, Greenhoot JH (1973) Responses evoked from the thalamic centrum medianum by painful input: suppression by dorsal funiculus conditioning. Exp Neurol 39:215–222
Ossipov MH, Sun H, Malan TP, Lai J, Porreca F (2000) Mediation of spinal nerve injury induced tactile allodynia by descending facilitatory pathways in the dorsolateral funiculus in rats. Neurosci Lett 20:129–132
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates. Academic Press, London
Reddy SVR, Maderdrut JL, Yaksh TL (1980) Spinal cord pharmacology of adrenergic agonist-mediated antinociception. J Pharmacol Exp Ther 213:525–533
Ren B, Linderoth B, Meyerson BA (1996) Effects of spinal cord stimulation on the flexor reflex and involvement of supraspinal mechanisms: an experimental study in mononeuropathic rats. J Neurosurg 84:244–249
Roberts MHT, Rees H (1986) The antinociceptive effects of stimulating the pretectal nucleus of the rat. Pain 25:83–93
Saadé NE, Jabbur SJ (2008) Nociceptive behavior in animal models for peripheral neuropathy: spinal and supraspinal mechanisms. Prog Neurobiol 86:22–47
Saadé NE, Jundi AS, Jabbur SJ, Banna NR (1982) Dorsal column input to inferior raphé centralis neurons. Brain Res 250:345–348
Saadé NE, Salibi NA, Banna NR, Towe AL, Jabbur SJ (1983) Spinal input pathways affecting the medullary gigantocellular reticular nucleus. Exp Neurol 80:582–600
Saadé NE, Tabet MS, Atweh SF, Jabbur SJ (1984) Modulation of segmental mechanisms by activation of a dorsal column brainstem spinal loop. Brain Res 310:180–184
Saadé NE, Tabet MS, Banna NR, Atweh SF, Jabbur SJ (1985) Inhibition of nociceptive evoked activity in spinal neurons through a dorsal column-brainstem-spinal loop. Brain Res 339:115–118
Saadé NE, Tabet MS, Soueidan SA, Bitar M, Atweh SF, Jabbur SJ (1986) Supraspinal modulation of nociception in awake rats by stimulation of the dorsal column nuclei. Brain Res 369:307–310
Saadé NE, Atweh SF, Jabbur SJ, Wall PD (1990) Effects of lesions in the anterolateral columns and dorsolateral funiculi on self-mutilation behavior in rats. Pain 42:313–321
Saadé NE, Atweh SF, Privat A, Jabbur SJ (1999) Inhibitory effects from various types of dorsal column and raphe magnus stimulations on nociceptive withdrawal flexion reflexes. Brain Res 846:72–86
Saadé NE, Baliki MN, El-Khoury C, Hawwa NN, Atweh SF, Apkarian AV, Jabbur SJ (2002) The role of the dorsal columns in neuropathic behavior: evidence for plasticity and non specificity. Neuroscience 115:403–413
Saadé NE, Al Amin H, Chalouhi S, Abdel Baki S, Jabbur SJ, Atweh SF (2006) Spinal pathways involved in supraspinal modulation of neuropathic manifestations in rats. Pain 126:280–293
Saadé NE, Al Amin H, Tchachaghian S, Jabbur SJ, Atweh SF (2010) Alteration of GABAergic and glycinergic mechanisms by lidocaine injection in the rostral ventromedial medulla of neuropathic rats. Pain 149:89–99
Saadé NE, Al Amin HA, Barchini J, Tchachaghian S, Shamaa F, Jabbur SJ, Atweh SF (2012) Brainstem injection of lidocaine releases the descending pain-inhibitory mechanisms in a rat model of mononeuropathy. Exp Neurol 237:180–190
Salibi NA, Saadé NE, Banna NR, Jabbur SJ (1980) Dorsal column input into the reticular formation. Nature 288:481–483
Sandkuhler J, Fu QG, Zimmermann M (1987) Spinal pathways mediating tonic or stimulation-produced descending inhibition from the periaqueductal gray or nucleus Raphé magnus are separate in the cat. J Neurophysiol 58:327–341
Shafizadeh M, Semnanian S, Zarrindast MR, Hashemi B (1997) Involvement of GABAB receptors in the antinociception induced by baclofen in the formalin test. Gen Pharmacol Vasc Syst 28:611–615
Song Z, Ultenius C, Meyerson BA, Linderoth B (2009) Pain relief by spinal cord stimulation involves serotonergic mechanisms: an experimental study in a rat model of mononeuropathy. Pain 147:241–248
Song Z, Meyerson BA, Linderoth B (2011) Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain 152:1666–1673
Song Z, Ansah OB, Meyerson BA, Pertovaara A, Linderoth B (2013a) The rostro ventromedial medulla is engaged in the effects of spinal cord stimulation in a rodent model of neuropathic pain. Neuroscience 247:133–144
Song Z, Ansah OB, Meyerson BA, Pertovaara A, Linderoth B (2013b) Exploration of supraspinal mechanisms in effects of spinal cord stimulation: role of the locus coeruleus. Neuroscience 253:426–434
Stiller CO, Linderoth B, O’Connor WT, Franck J, Falkenberg T, Ungerstedt U, Brodin E (1995) Repeated spinal cord stimulation decreases the extracellular level of gamma-aminobutyric acid in the periaqueductal gray matter of freely moving rats. Brain Res 699:231–241
Tanaka M, Matsumoto Y, Murakami T, Hisa Y, Ibata Y (1996) The origins of catecholaminergic innervation in the rostral ventromedial medulla oblongata of the rat. Neurosci Lett 207:53–56
Tsuruoka M, Maeda M, Nagasawa I, Inoue T (2004) Spinal pathways mediating coeruleospinal antinociception in the rat. Neurosci Lett 362:236–239
Ultenius C, Song Z, Lin P, Meyerson BA, Linderoth B (2013) Spinal GABAergic mechanisms in the effects of spinal cord stimulation in a rodent model of neuropathic pain: is GABA synthesis involved? Neuromodulation 16:114–120
Villanueva L, Chitour D, Le Bars D (1986) Involvement of the dorsolateral funiculus in the descending spinal projections responsible for diffuse noxious inhibitory controls in the rat. J Neurophysiol 56:1185–1195
Voisin DL, Guy N, Chalus M, Dallel R (2005) Nociceptive stimulation activates locus coeruleus neurones projecting to the somatosensory thalamus in the rat. J Physiol 566:929–937
Wall PD, Bery J, Saadé NE (1988) Effects of lesion to rat spinal cord lamina I cell projection pathways on reactions to acute and chronic noxious stimuli. Pain 35:327–339
Wei H, Viisanen H, Pertovaara A (2009) Descending modulation of neuropathic hypersensitivity by dopamine D2 receptors in or adjacent to the hypothalamic A11 cell group. Pharamacol Res 59:355–363
Zimmermann M (1983) Ethical guidelines for investigation of experimental pain in conscious animals. Pain 16:109–110
Acknowledgments
The authors thank Sawsan Sharrouf and Bassem Najm for their technical assistance. This work was supported by a grant from the Swedish Research Council. Formal approval to conduct the experiments described has been obtained from the Animal Care Facility at the American University of Beirut and could be provided upon request. All efforts were made to minimize the number of animals used and their suffering.
Conflict of interest
There are no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saadé, N.E., Barchini, J., Tchachaghian, S. et al. The role of the dorsolateral funiculi in the pain relieving effect of spinal cord stimulation: a study in a rat model of neuropathic pain. Exp Brain Res 233, 1041–1052 (2015). https://doi.org/10.1007/s00221-014-4180-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-014-4180-x