Abstract
-
1.
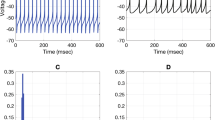
The conductance and kinetics of the Ca2+ activated K+ channels were studied in voltage clampedHelix neurones by using noise and relaxation techniques.
-
2.
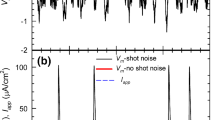
The increase of outward current activated by the injection of Ca2+ ions into the cells is associated with an increase of membrane current fluctuations. The spectral density of the K+ current fluctuations decays at high frequency quency with an overall slope of aboutf −1.5. Most of the spectra can be fitted by double Lorentzian curves. The single channel conductance derived from integrated powder spectra is 12–16 pS.
-
3.
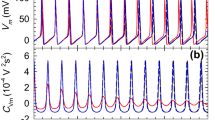
Voltage jump experiments show that the gating of the Ca2+ activated K+ current follows first order kinetics provided that the currents are small. The time constant is voltage dependent and increases about e-fold per 85 mV membrane depolarization. Its magnitude isindependent of the previous membrane potential.
-
4.
The instantaneous current-voltage relation of the Ca2+ activated K+ current is non-linear and can be fitted by the constant field relation. The steady-state current-voltage relation exhibits stronger rectification than the instantaneous current-voltage relation and follows the constant field relation with voltage dependent permeability coefficient. The voltage dependence of the permeability coefficient is the same as that of the relaxation time constant.
-
5.
The voltage dependent increase of the K+ conductance together with the voltage dependent increase of the time constant suggests that the effective open time of the ion channel is prolonged by a decrease in the backward rateconstants determining the transition to the closed state, i.e. either the channel life-time or the unbinding rate-constant of Ca2+. The opening rate-constant appears to be voltage independent.
Similar content being viewed by others
References
Adams PR, Constanti A, Clark RB, Adams CEY and Brown DA (1981) Single channel and noise analysis of outward currents in bullfrog ganglion cells. Soc Neurosci Abstr 7:15
Anderson CR, Stevens CF (1973) Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol 235:655–691
Anderson CR, Cull-Candy SG, Miledi R (1976) Glutamate and quisqualate noise in voltage-clamped muscle fibres. Nature 261:151–153
Ascher P, Marty A, Neild TO (1978) Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol 178:177–206
Bernasconi CF (1976) Relaxation kinetics. Academic Press, New York
Colquhoun D, Hawkes AG (1977) Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B 199:231–262
Conti F, Neher E (1980) Single channel recordings of K+ currents in squid axons. Nature 285:140–143
Dionne VE (1981) The kinetics of slow muscle acetylcholine-operated channels in the garter snake, J Physiol 310:159–190
Dreyer F, Walther Chr, Peper K (1976) Junctional and extrajunctional acetylcholine receptors in normal and denervated frog muscle fibres. Pflügers Arch 366:1–9
Dudel J, Finger W, Stettmeier H (1980) Inhibitory synaptic channels activated by γ-aminobutyric acid (GABA) in crayfish muscle. Pflügers Arch 387:143–151
Eckert R, Lux HD (1976) A voltage-sensitive persistant calcium conductance in neuronal somata of Helix. J Physiol 254:129–151
Goldman DE (1943) Potential, impedance, and rectification in membranes. J Gen Physiol 27:37–60
Gorman ALF, Thomas MV (1980) Potassium conductance and internal calcium accumulation in a molluscan neurone. J Physiol 308:287–313
Gorman ALF, Hermann A, Thomas MV (1981) Intracellular calcium and the control of neuronal pacemaker activity. Fed Proc 40:2233–2239
Hartung K, Hermann A (1981) Kinetics and current voltage relations of the calcium-activated potassium current in Helix neurones. Pflügers Arch 391:R35
Hermann A, Hartung K (1981) Ca2+ activated K+ current noise and relaxation measurements in Helix neurones. Soc Neurosci Abstr 7:14
Hermann A, Hartung K (1982) Properties of Ca2+ activated K+ conductance in Helix neurones investigated by intracellular Ca2+ ionophoresis. Pflügers Arch 393:248–253
Hodgkin AL, Katz B (1949) The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol 108:37–77
Hofmeier G, Lux HD (1981) Three distinct effects mediated by calcium ions on electrical membrane properties of Helix neurons. Proc XXVIII Intern Congress Physiol Sci, Akadémiai Kiadó, Budapest
Lux HD (1980) Voltage dependence of Ca2+-activated K+ conductance. In: Koester J, Byrne JH (eds) Molluscan nerve cells. From biophysics to behavior. Cold Spring Harbor Laboratory 1:105–114
Lux HD, Neher E, Marty A (1981) Single channel activity associated with the calcium dependent outward current in Helix pomatia. Pflügers Arch 389:293–295
Magleby KL, Stevens CF (1972) The effect of voltage on the time course of end-plate currents. J Physiol 223:151–171
Marchais D, Marty A (1979) Interaction of permeant ions with channels activated by acetylcholine in Aplysia neurones. J Physiol 297:9–45
Marty A (1981) Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature 291:497–500
McLaughlin SGA, Szabo G, Eisenman G (1971) Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol 58:667–687
Meech RW (1978) Calcium-dependent potassium activation in nervous tissues. Ann Rev Biophys Bioeng 7:1–18
Meech RW (1980) Ca2+-activated K+ conductance. In: Koester J, Byrne JH (eds) Molluscan nerve cells. From biophysics to behavior. Cold Spring Harbor Laboratory 1:93–103
Neher E, Sakmann B (1975) Voltage-dependence of drug-induced conductance in frog neuromuscular junction. Proc Natl Acad Sci USA 72:2140–2144
Neher E, Stevens CF (1977) Conductance fluctuations and ionic pores in membranes. Ann Rev Biophys Bioeng 6:345–381
Neher E, Sakmann B, Steinbach JH (1978) The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflügers Arch 375:219–228
Nelson DJ, Sachs F (1979) Single ionic channels observed in tissue-cultured muscle. Nature 282:861–863
Noma A, Trautwein W (1978) Relaxation of the ACh-induced potassium current in the rabbit sinoatrial node cell. Pflügers Arch 377:193–200
Pallotta BS, Magleby KL, Barrett JN (1981) Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature 293:471–474
Rose B, Loewenstein WR (1975) Calcium ion distribution in cytoplasm visualized by aequorin: diffusion in cytosol restricted by energized sequestering. Science 190:1204–1206
Sakmann B, Patlak J, Neher E (1980) Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature 286:71–73
Sheridan RE, Lester HA (1977) Rates and equilibria at the acetylcholine receptor of electrophorus electroplaques. A study of neurally evoked postsynaptic currents and of voltage-jump relaxations. J Gen Physiol 70:187–219
Woolum JC, Gorman ALF (1981) Time dependence of the calcium-activated potassium current. Biophys J 36:297–302
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hermann, A., Hartung, K. Noise and relaxation measurements of the Ca2+ activated K+ current in Helix neurones. Pflugers Arch. 393, 254–261 (1982). https://doi.org/10.1007/BF00584079
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00584079