Summary
-
1.
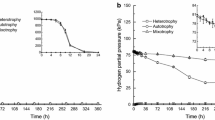
When metronidazole (1-β-hydroxyethyl-2-methyl-5-nitroimidazole) was added at 50 μg/ml to a liquid (35°C) culture of Clostridium acetobutylicum (0.5 mg dry wt of organisms/ml), it inhibited growth for only 2 h; thereafter, growth resumed at a slower and arithmetic rate. Consumption of glucose and excretion of acetate plus butyrate continued, though at diminished rates, during the period of bacteriostasis, and the ATP content of the organisms did not decline.
-
2.
Metronidazole inhibited production of H2 by a culture of Cl. acetobutylicum, yet it only temporarily halted formation of H2 from pyruvate by cell extracts, and had no deleterious effect on the simultaneous production of acetyl phosphate and CO2. After a relatively short time no residual metronidazole was detectable in this cell-free system and H2 production resumed at its former rate.
-
3.
The drug did not inhibit the hydrogenases of either Cl. acetobutylicum or Cl. pasteurianum, but having an E′ 0 at pH 7 of approx. -0.23 V (on the hydrogen scale), it acted as a preferential acceptor of electrons from reduced ferredoxin (or reduced viologen dyes). Reduction of metronidazole was a 6 electron process compatible with complete reduction of its 5-nitro group. Yet, although the expected decrease in absorbance at 320 nm was observed, the 5-amino derivative did not accumulate (probably due to its intrinsic lability).
-
4.
Since many aryl nitro compounds are similarly able to accept electrons from reduced ferredoxin, it seems that additional reasons might have to be sought to explain the unique effectiveness of metronidazole as an antimicrobial agent.
Similar content being viewed by others
References
Breinlich, J.: Zur Analyse arzeneilich gebrauchter Nitrofurane und des Metronidazols. Dtsch. Apoth.-Ztg. 104, 535–540 (1964).
Buchanan, B. B., Lovenberg, W., Rabinowitz, J. C.: A comparison of clostridial ferredoxins. Proc. nat. Acad. Sci. (Wash.) 49, 345–353 (1963).
Edwards, D. I., Mathison, G. E.: The mode of action of metronidazole against Trichomonas vaginalis. J. gen. Microbiol. 63, 297–302 (1970).
Fargher, R. G.: Orientation of the nitro- and arylazoglyoxalines. Fission of the glyoxaline nucleus. J. chem. Soc. 117, 668–680 (1920).
Forrest, W. W., Walker, D. J.: Synthesis of reserve materials for endogenous metabolism in Streptococcus faecalis. J. Bact. 89, 1448–1452 (1965).
Freeman, W. A., McFadzean, J. A., Whelan, J. P. F.: Activity of metronidazole against experimental tetanus and gas gangrene. J. appl. Bact. 31, 443–447 (1968).
Kornberg, H. L., Morris, J. G.: The utilization of glycollate by Micrococcus denitrificans; the β-hydroxyaspartate pathway. Biochem. J. 95, 577–586 (1965).
Lipmann, F., Tuttle, L. C.: A specific micromethod for the determination of acyl phosphates. J. biol. Chem. 159, 21–28 (1945).
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J.: Protein measurement with the Folin phenol reagent. J. biol. Chem. 193, 265–275 (1951).
McCalla, D. R., Reuvers, A., Kaiser, C.: Mode of action of nitrofurazone. J. Bact. 104, 1126–1134 (1970).
O'Brien, R. W., Morris, J. G.: The ferredoxin-dependent reduction of chloramphenicol by Clostridium acetobutylicum. J. gen. Microbiol. 67, 265–271 (1971a).
——: Oxygen and the growth and metabolism of Clostridium acetobutylicum. J. gen. Microbiol. 68, 307–318 (1971b).
Prince, H. N., Grunberg, E., Titsworth, E., DeLorenzo, W. F.: Effects of 1-(2-nitro-1-imidazolyl)-3-methoxy-2-propanol and 2-methyl-5-nitroimidazole-1-ethanol against anaerobic and aerobic bacteria and protozoa. Appl. Microbiol. 18, 728–730 (1969).
Stambaugh, J. E., Manthei, R. W.: The characterization of substituted nitroimidazoles on paper and thin layer chromatography, by colorimetric reactions. J. Chromat. 31, 128–136 (1967).
Stanley, P. E., Williams, S. G.: Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Analyt. Biochem. 29, 381–392 (1969).
Teller, J. D.: Direct, quantitative, colorimetric determination of serum or plasma glucose. Abstracts of Papers, 130th Meeting Amer. Chem. Soc., p. 69c (1956).
Umbreit, W. W., Burris, R. H., Stauffer, J. F.: Manometric techniques. Minneapolis: Burgess Publ. Co. 1964.
Valentine, R. C.: Bacterial ferredoxin. Bact. Rev. 28, 497–517 (1964).
Vignoli, L., Cristau, B., Gouezo, F., Fabre, C.: Étude polarographique de quelques composés hétérocycliques nitrés. Chim. Analyt. 45, 439–445 (1963).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
O'Brien, R.W., Morris, J.G. Effect of metronidazole on hydrogen production by Clostridium acetobutylicum . Archiv. Mikrobiol. 84, 225–233 (1972). https://doi.org/10.1007/BF00425200
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00425200