Abstract
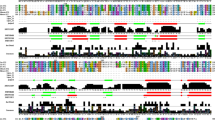
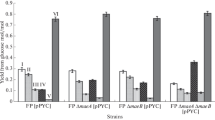
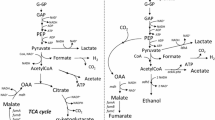
Four classes of Escherichia coli mutants deficient in either or both of their anaerobic selenium-containing formate dehydrogenases (FDH) were isolated. A class I mutant devoid of FDHH activity specifically linked to benzyl viologen (BV) produced a small amount of the FDHH 80,000 dalton selenopeptide. Three class II mutants were deficient in FDHN activity specifically linked to phenazine methosulfate (PMS) and exhibited a selenopeptide “doublet” rather than the FDHN 110,000 dalton selenosubunit. Three class III mutants were selenium incorporation deficient and did not exhibit either FDH activity or 75Selabeled selenopolymers. A class IV mutant was devoid of PMS-linked FDHN activity; neither its FDHN 110,000 dalton selenosubunit nor its BV-linked FDHH activity was fully regulated by nitrate.
Similar content being viewed by others
Abbreviations
- FDH:
-
formate dehydrogenase
- BV:
-
benzyl viologen
- MV:
-
methyl viologen
- PMS:
-
phenazine methosulfate
- SDS-PAGE:
-
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
References
Adelberg EA, Mandel M, Chen GCC (1965) Optimal conditions for mutagenesis by N-methyl-N′-nitro-N-nitrosoguanidine. Biochem Biophys Res Commun 18:788–795
Barrett EL, Jackson CE, Fukumoto HT, Chang GW (1979) Formate dehydrogenase mutants of Salmonella typhimurium: a new medium for their isolation and new mutant classes. Mol Gen Genet 177:95–101
Chaudhry GH, Chaiken IM, MacGregor, CH (1983) An activity from Escherichia coli membranes responsible for the modification of nitrate reductase to its precursor form. J Biol Chem 258:5828–5833
Ching W-M, Tsai L, Wittwer AJ (1985) Selenium-containing transfer RNAs. Curr Top Cell Regul 27:497–507
Cox JC, Edwards ES, DeMoss JA (1981) Resolution of distinct selenium-containing formate dehydrogenases from Escherichia coli. J Bacteriol 145:1317–1324
Enoch HG, Lester RL (1975) The purification and properties of formate dehydrogenase and nitrate reductase from Escherichia coli. J Biol Chem 250:6693–6705
Glaser JH, DeMoss JA (1971) Phenotypic restoration by molybdate of nitrate reductase activity in chlD mutants of Escherichia coli. J Bacteriol 108:854–860
Graham AG, Boxer DH (1981) The organization of formate dehydrogenase in the cytoplasmic membrane of Escherichia coli. Biochem J 195:627–637
Hubbard S, Ivatt RJ (1981) Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem 50:555–583
Ingledew WJ, Poole RK (1984) The respiratory chains of Escherichia coli. Microbiol Rev 48:222–271
Kramer GF, Ames BN (1988) Isolation and characterization of a selenium metabolism mutant of Salmonella typhimurium. J Bacteriol 170:736–743
Leinfelder W, Forchhammer K, Zinoni F, Sawers G, Mandrand-Berthelot M-A, Bock A (1988) Escherichia coli genes whose products are involved in selenium metabolism. J Bacteriol 170:540–546
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurements with the Folin phenol reagent. J Biol Chem 193:265–275
Pecher A, Zinoni F, Bock A (1985) The selenopolypeptide of formic dehydrogenase (formate hydrogen-lyase linked) from Escherichia coli: genetic analysis. Arch Microbiol 141:359–363
Revis C (1912) The production of variation in the physiological activity of Bacillus coli by the use of malachite green. Proc R Soc Lond, Ser B, 85:192–194
Segrest, JP, Jackson RL, Andrews EP, Marchesi VT (1971) Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun 44:390–395
Tyhach R, Hawrot E, Satre M, Kennedy E (1979) Increased synthesis of phosphatidylserine decarboxylase in a strain of Escherichia coli bearing a hybrid plasmid. J Biol Chem 254:627–633
Wold F (1981) In vivo chemical modification of proteins (post-translational modification). Annu Rev Biochem 50:783–814
Wu LF, Mandrand-Berthelot M-A (1987) Regulation of the fdhF gene encoding the selenopeptide for benzyl viologen-linked formate dehydrogenase in Escherichia coli. Mol Gen Genet 209:129–134
Zinoni F, Birkmann A, Stadtman TC, Bock A (1986) Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc Natl Acad Sci USA 83:4650–4654
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cox, J.C. Escherichia coli formate dehydrogenase mutants with altered selenopolymer profiles. Arch. Microbiol. 152, 397–400 (1989). https://doi.org/10.1007/BF00425180
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00425180