Summary
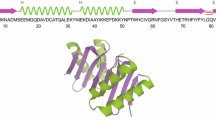
Nearly complete assignment of the protonated carbon resonances of apo-neocarzinostatin, 113-amino acid antitumor antibiotic carrier protein, has been achieved at natural 13C abundance using heteronuclear 2D experiments. Most of the cross peaks in the proton-carbon correlation map were identified by the combined use of HMQC, HMQC-RELAY and HMQC-NOESY spectra, using already published proton chemical shifts. However, double-DEPT and triple-quantum experiments had to be performed for the edition of CH and CH2 side-chain groups, respectively, which were hardly visible on HMQC-type maps. The triple-quantum pulse sequence was adapted from its original scheme to be applicable to a natural abundance sample. The correlation between carbon chemical shifts and the apo-neocarzinostatin structure is discussed. In particular, 13C alpha secondary shifts correlate well with the backbone conformation. These shifts also yield information about the main-chain flexibility of the protein. Assignments reported herein will be used further for interpretation of carbon relaxation times in a study of the internal dynamics of apo-neocarzinostatin.
Similar content being viewed by others
References
AdjadjE., MispelterJ., QuiniouE., DimicoliJ.L., FavaudonV. and LhosteJ.M. (1990) Eur. J. Biochem., 190, 263–271.
AdjadjE., QuiniouE., MispelterJ., FavaudonV. and LhosteJ.M. (1992a) Eur. J. Biochem., 203, 505–511.
AdjadjE., QuiniouE., MispelterJ., FavaudonV. and LhosteJ.M. (1992b) Biochimie, 74, 853–858.
BaxA., GriffeyR.H. and HawkinsB.L. (1983) J. Magn. Reson., 55, 301–315.
BaxA., IkuraM., KayL.E., TorchiaD.A. and TschudinR. (1990) J. Magn. Reson., 86, 304–318.
BendallM.R., PeggD.T. and DoddrellD.M. (1983) J. Magn. Reson., 52, 81–117.
BodenhausenG. and RubenD.J. (1980) Chem. Phys. Lett., 69, 185–189.
BrühwilerD. and WagnerG. (1986) J. Magn. Reson., 69, 546–551.
CloreG.M., BaxA., DriscollP.C., WingfieldP.T. and GronenbornA.M. (1990) Biochemistry, 29, 8172–8184.
DavisD.G. (1990) J. Magn. Reson., 90, 589–596.
DavisD.G. (1991) J. Magn. Reson., 91, 665–672.
deDiosA.C., PearsonJ.G. and OldfieldE. (1993) Science, 260, 1491–1496.
DoddrellD.M., PeggD.T. and BendallM.R. (1982) J. Magn. Reson., 48, 323–327.
DomkeT. and LeibfritzD. (1990) J. Magn. Reson., 88, 401–405.
ErnstR.R., BodenhausenG. and WokaumA. (1987) In International Series of Monographs on Chemistry, Vol. 14 (Eds, BreslowR., HalpernJ. and RowlinsonJ.S.) Clarendon Press, Oxford, pp. 467–489.
GaoX. and BurkhartW. (1991) Biochemistry, 30, 7730–7739.
GaoX. (1992) J. Mol. Biol., 225, 125–135.
HowarthO.W. and LilleyD.M.J. (1978) Prog. NMR Spectrosc., 12, 1–40.
IkuraM., SperaS., BarbatoG., KayL.E., KrinksM. and BaxA. (1991) Biochemistry, 30, 9216–9228.
KappenL.S., NapierM.A. and GoldbergI.H. (1980) Proc. Natl. Acad. Sci. USA, 77, 1970–1974.
KayL.E., JueT.L., BangerterB. and DemouP.C. (1987) J. Magn. Reson., 73, 558–564.
KayL.E. and BaxA. (1989) J. Magn. Reson., 84, 598–603.
KesslerH., SchmiederP., and KurzM. (1989) J. Magn. Reson., 85, 400–405.
KimK.H., KwonB.M., MyersA.G. and ReesD.C. (1993) Science, 262, 1042–1046.
LawsD.D., deDiosA.C. and OldfieldE. (1993) J. Biomol. NMR, 3, 607–612.
LernerL. and BaxA. (1986) J. Magn. Reson., 69, 375–380.
LondonR.E. and AvitabileJ. (1978) J. Am. Chem. Soc., 100, 7159–7165.
MüllerL. (1979) J. Am. Chem. Soc., 101, 4481–4484.
NirmalaN.R. and WagnerG. (1988) J. Am. Chem. Soc., 110, 7557–7558.
NortonR.S., ClouseA.O., AddlemanR. and AllerhandA. (1977) J. Am. Chem. Soc., 99, 79–83.
NorwoodT.J., BoydJ., HeritageJ.E., SoffeN. and CampbellI.D. (1990) J. Magn. Reson., 87, 488–501.
OhB.H., WestlerW.M. and MarkleyJ.L. (1989) J. Am. Chem. Soc., 111, 3083–3085.
OldfieldE., NortonR.S. and AllerhandA. (1975) J. Biol. Chem., 250, 6368–6380.
PalmerIIIA.G., CavanaghJ., WrightP.E. and RanceM. (1991) J. Magn. Reson., 93, 151–170.
PastoreA. and SaudekV. (1990) J. Magn. Reson., 90, 165–176.
RemerowskiM.L., GlaserS.J., SiekerL.C., SamyT.S.A. and DrobnyG.P. (1990) Biochemistry, 29, 8401–8409.
RibeiroA.A., KingR., RestivoC. and JardetzkyO. (1980) J. Am. Chem. Soc., 102, 4040–4051.
RicharzR., NagayamaK. and WüthrichK. (1980) Biochemistry, 19, 5189–5196.
SchmidtJ.M. and RüterjansH. (1990) J. Am. Chem. Soc., 112, 1279–1280.
ShakaA.J., KeelerJ., FrenkielT. and FreemanR. (1983) J. Magn. Reson., 52, 335–338.
ShonK. and OpellaS.J. (1989) J. Magn. Reson., 82, 193–197.
SklenarV., TorchiaD. and BaxA. (1987) J. Magn. Reson., 73, 375–379.
SørensenO.W., EichG.W., LevittM.H., BodenhausenG. and ErnstR.R. (1983) Prog. NMR Spectrosc., 16, 163–192.
SperaS. and BaxA. (1991) J. Am. Chem. Soc., 113, 5490–5492.
SummersM.F., MarzilliL.G. and BaxA. (1986) J. Am. Chem. Soc., 108, 4285–4294.
TateS.I., MasuiY. and InagakiF. (1991) J. Magn. Reson., 94, 625–630.
TeplyakovA., ObmolovaG., WilsonK. and KuromizuK. (1993) Eur. J. Biochem., 213, 737–741.
WagnerG. and BrühwilerD. (1986) Biochemistry, 25, 5839–5843.
WagnerG. (1989) Methods Enzymol., 176, 93–113.
WishartD.S., SykesB.D. and RichardsF.M. (1991) J. Mol. Biol., 222, 311–333.
WüthrichK. (1976) NMR in Biological Research: Peptides and Proteins, Elsevier, New York, NY, pp. 157–209 and 293–316.
ZhangX. and WangC. (1991) J. Magn. Reson., 91, 618–623.
ZuiderwegE.R.P. (1990) J. Magn. Reson., 86, 346–357.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lefevre, C., Adjadj, É., Quiniou, É. et al. Assignment of the protonated 13C resonances of apo-neocarzinostatin by 2D heteronuclear NMR spectroscopy at natural abundance. J Biomol NMR 4, 689–702 (1994). https://doi.org/10.1007/BF00404278
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00404278