Summary
-
1.
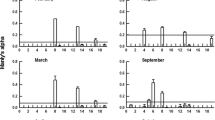
Adult females of the predaceous copepod, Diacyclops thomasi, consistently selected for the soft-bodied rotifers Synchaeta pectinata, Polyarthra major and P. remata when presented various combinations of 8 rotifer species and 2 crustacean species as prey. Diacyclops did not select for other small, soft-bodied rotifers such as P. vulgaris and Ascomorpha ecaudis and, for loricate species such as Keratella cochlearis, K. crassa and for large soft-bodied adult Asplanchna priodonta. The small cladocerans, Bosmina longirostris and Chydorus sphaericus also were resistant to predation by this copepod.
-
2.
Increased hunger in Diacyclops increased the clearance rates on both vulnerable and Diacyclops-resistant prey but did not greatly increase mortality of resistant prey relative to vulnerable prey. Sated Diacyclops preferred small, vulnerable prey like P. major over larger-bodied Synchaeta. This effect may be attributed to limited gut space when food is abundant.
-
3.
When Diacyclops was presented different relative proportions of Keratella and Synchaeta at a constant total prey density (500 prey/L), it selected Synchaeta over Keratella in all trial proportions. However, Diacyclops selected more strongly for Keratella (but at a much lower clearance rate than for Synchaeta) when the relative abundance of this predator-resistant species was greatest. These results support optimal foraging in this predator.
-
4.
Predator-prey interactions of the kind reported in this study can help identify important food web pathways and can be used to interpret predator-mediated changes in zooplankton communities in mature.
Similar content being viewed by others
References
Brandl Z, Fernando CH (1978) Prey selection by the cyclopoid copepods Mesocyclops edax and Cyclops vicinus. Verh Internat Verein Limnol 20:2505–2510
Beachamp PD de (1932) Contribution a l'etude du genre Ascomorpha et des processus digestifs chez les rotiferes. Bull Soc Zool France 57:428–449
Drenner RW, deNoyelles F, Kettle D (1982) Selective impact of filter-feeding gizzard shad on zooplankton community structure. Limnol Oceanogr 27:965–968
Duncan A (1983) The composition, density and distribution of the zooplankton in Parakrama Samudra. In: Schiemer F (ed) Limnology of Parakrama Samudra-Sri Lanka: a case study of an ancient man-made lake in the tropics. Dr. W. Junk, The Hague, pp 85–94
Duncan A (1984) Assessment of factors influencing the composition, body size and turnover rate of zooplankton in Parakrama Samudra, an irrigation reservoir in Sri Lanka. Hydrobiol 113:201–215
Gannon JE (1972) A contribution to the ecology of zooplankton Crustacea of Lake Michigan and Green Bay. PhD Dissertation, University of Wisconsin-Madison
Gauld DT (1951) The grazing rate of planktonic copepods. J Mar Biol Assoc UK 29:695–706
Gerritsen J, Strickler JR (1977) Encounter probabilities and community structure in zooplankton: A mathematical model. J Fish Res Bd Can 34:73–82
Gilbert JJ, Williamson CE (1978) Predator-prey behavior and its effect on rotifer survival in associations of Mesocyclops edax, Asplanchna girodi, Polyarthra vulgaris, and Keratella cochlearis. Oecologia 37:13–22
Holling CS (1966) The functional response of invertebrate predators to prey density. Mem Ent Soc Can 48:1–86
Hrbáček J (1962) Species composition and the amount of zooplankton in relation to the fish stocks. Rozpr Crsk Akad Ved 72:1–116
Johnson DM, Akre BG, Crowley PH (1975) Modeling arthropod predation: wastefull killing by damselfly naiads. Ecol 56:1081–1093
Kerfoot WC (1977) Implications of copepod predation. Limnol Oceanogr 22:316–325
Kerfoot WC (1978) Combat between predatory copepods and their prey: Cyclops, Epischura and Bosmina. Limnol Oceanogr 23:1089–1102
Kerfoot WC (1982) A question of taste: crypsis and warning coloration in freshwater zooplankton communities. Ecol 63:538–554
Lewis WM Jr (1977) Feeding selectivity of a tropical Chaoborus population. Freshwat Biol 7:311–325
Li JL, Li HW (1979) Species-specific factors affecting predator-prey interactions of the copepod Acathocyclops vernalis with its natural prey. Limnol Oceanogr 24:613–626
Nauwerck A (1978) Notes on the planktonic rotifers of Lake Ontario. Arch Hydrobiol 84:269–301
Nichols HW (1973) Growth media-freshwater. In: Stein JR (ed) Handbook of Phycological Methods. Cambridge University Press, New York, pp 7–24
Norden CR (1968) Morphology and food habits of the larval alewife, Alosa pseudoharengus (Wilson), in Lake Michigan. Proc 11th Conf Great Lakes Res Internat Assoc Great Lakes Res 1968:103–110
O'Brien WJ (1979) The predator-prey interaction of planktivorous fish and zooplankton. Amer Sci 67:572–581
O'Brien WJ, Kette D, Riessen HP (1979) Helmets and invisible armor: structures reducing predation from tactile and visual planktivores. Ecol 60:287–294
Pastorok RA (1980) The effects of predator hunger and food abundance on prey selection by Chaoborus larvae. Limnol Oceanogr 25:910–920
Pastorok, RA (1981) Prey vulnerability and size selection by Chaoborus larvae. Ecol 62:1311–1324
Pyke GH, Pulliam HR, Charnov EL (1977) Optimal foraging: a selective review of theory and tests. Quart Rev Biol 52:137–154
Riessen HP (1980) Diel vertical migration of pelagic water mites. In: Kerfoot WC (ed) The evolution and ecology of zooplankton communities. Univ Press of New England, pp 129–137
Siefert RE (1972) First food of larval yellow perch, bluegill, emerald shiner and rainbow smelt. Trans Am Fish Soc 101:219–225
Stemberger RS (1974) Temporal and spatial distributions of planktonic rotifers in Milwaukee Harbor and adjacent Lake Michigan. Proc 17th Conf Great Lakes Res, Internat Assoc Great Lakes Res, pp 120–134
Stemberger RS (1981) A general approach to the culture of planktonic rotifers. Can J Fish Aquat Sci 38:721–724
Stemberger RS, Evans MS (1984) Rotifer seasonal succession and copepod predation in Lake Michigan. J Great Lakes Res 10:417–428
Stemberger RS, Gilbert JJ (1984) Spine development in the rotifer Keratella cochlearis: induction by cyclopoid copepods and Asplanchna. Freshwat Biol, in press
Stemberger RS, Gilbert JJ Body size, food concentration and population growth in planktonic rotifers. Ecol (in press)
Stemberger RS, Gannon JE, Bricker FJ (1979) Spacial and seasonal structure of rotifer communities in Lake Huron. EPA-600/3-79-085
Vanderploeg HA, Scavia D (1979) Two electivity indices for feeding with special reference to zooplankton grazing. J Fish Res Board Can 36:362–365
Williamson CE (1980) The predatory behavior of Mesocyclops edax: predator preferences, prey defenses, and starvation induced changes. Limnol Oceanogr 25:903–909
Williamson CE (1983) Invertebrate predation on planktonic rotifers. Hydrobiol 104:385–396
Zaret TM (1972) Predators, invisible prey, and the polymorphism in the Cladocera (Class Crustacea). Limnol Oceanogr 17:171–184
Zaret TM, Kerfoot WC (1975) Fish predation on Bosmina longirostris: visibility selection versus body-size selection. Ecol 56:232–237
Author information
Authors and Affiliations
Additional information
This study was partially supported by NOAA contract NA81-RAH00003 to J.A. Bowers, The University of Michigan. Contribution number 408 Great Lakes Research Division, The University of Michigan
Rights and permissions
About this article
Cite this article
Stemberger, R.S. Prey selection by the copepod Diacyclops thomasi . Oecologia 65, 492–497 (1985). https://doi.org/10.1007/BF00379662
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00379662