Summary
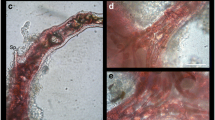
Specimens of the sponge Halichondria panicea Pallas kept in running sea-water aquaria at 15° C slough off their complete outer tissue layer in regular intervals of three weeks. Sloughing starts at the rim of the oscula and extends over the whole surface within two weeks. Microscopic inspection of the tissue flakes shows them to harbour large numbers of different live organisms, as well as biogenic debris such as pieces of copepod carapaces and diatom frustules. No such community is found on freshly sloughed sponge tissue. After sloughing, the surface skeletal structure of H. panicea is markedly altered, as the characteristic halichondroid reticulum has been replaced by the irregular spicule array typical for the inner sponge tissue. When H. panicea is kept in closed aquaria filled with 0.2 μm filtered sea-water, no sloughing occurs during 3 months of maintenance. As those sponges shedding their outer tissue grow steadily at the same time, the tissue sloughing can be regarded as a reaction to sedimentation of organic material and settlement of small organisms on the sponge surface. The sponge thus counteracts clogging of its ostia and prevents the establishment of a micro fouling community on its surface, inhibiting further fouling processes.
Similar content being viewed by others
References
Anger K (1972) Dipurena spongicola sp. n. (Hydrozoa, Corynidae), ein in Schwämmen lebender Hydroidpolyp aus dem Kattegat und der nördlichen Kieler Bucht. Kieler Meeresforsch 28:80–83
Bakus GJ, Targett NM, Schulte B (1986) Chemical ecology of marine organisms: an overview. J Chem Ecol 12:951–987
Barthel D (1988) Growth of the sponge Halichondria panicea in a North Sea habitat. Proceedings of the 21st European Marine Biology Symposium 1986, Gdansk, Poland (in press)
Barthel D, Theede H (1986) A new method for the culture of marine sponges and its application for experimental studies. Ophelia 25(2):75–82
Bongers T (1983) Bionomics and reproductive cylce of the nematode Leptosomatum bacillatum living in the sponge Halichondria panicea. Neth J Sea Res 17(1):39–46
Burton M (1948) Observations on sponges, including the supposed swarming of larvae, movement and coalescence in mature individuals, longevity and death. Proc Zool Soc London 118:893–915
Christensen T (1985) Microspora ficulinae, a green alga living in marine sponges. Br Phycol J 20:5–7
Connes R (1967) Réactions de défense de l'éponge Thethya lyncurium Lamarck, vis-á-vis des micro-organismes et de l'amphipode Leucothoe spinicarpa Abildg. Vie et milieu 18A(2):281–289
Ciereszko LS, Mizelle JW, Schmidt RW (1973) Occurrence of taurobetaine in coelenterates and of polysaccharide sulfate in the gorgonian Pseudoterogorgia americana. In: Worthen LR (ed) Food-Drugs from the Sea Proceedings 1972. Marine Technology Society, Washington, D.C.
Conover JT, Sieburth JM (1964) Effect of Sargassum distribution on its epibiota and antibacterial activity. Bot Mar 6:147–155
Costello MJ, Myers AA (1987) Amphipod fauna of the sponges Halichondria panicea and Hymeniacidon perleve in Lough Hine, Ireland. Mar Ecol Prog Ser 41:115–121
Eimhjellen KE (1967) Photosynthetic bacteria and carotenoids from a sea sponge Halichondrium panicea. Acta Chem Scand 21:2281–2281
Filion-Myklebust C, Norton TA (1981) Epidermis shedding in the brown seaweed Ascophyllum nodosum (L.). Le Jolis, and its ecological significance. Mar Biol Lett 2:45–51
Fischer H (1978) Hydroids in biotest:Clava multicornis exposed to cadmium. Kieler Meeresforsch [Suppl 4]:327–334
Firth DW (1977) A preliminary analysis of the association of amphipods Microdeutopus damnoniensis (Bate), M. anomalus (Rathke) and Corophium sextoni Crawford with the sponges Halichondria panicea (Pallas) and Hymeniacidon perleve (Montagu). Crustaceana 32:113–118
Fuller JI (1946) Season of attachment and growth of sedentary marine organisms at Lamoine, Maine. Ecology 27:150–158
Graham HW, Gay H (1945) Season of attachment and growth of sedentary marine organisms at Oakland, California. Ecology 26:375–386
Hummel H, Sepers ABJ, Wolf L de, Melissen FW (1988) Bacterial growth on the marine sponge Halichondria panicea induced by reduced waterflow rate. Mar Ecol Prog Ser 42:195–198
Jagels RH (1970) Cell wall development in a marine monocotyledon. Am J Bot [Abstr] 57:737–738
Johnson CR, Mann KH (1986) The crustose coralline alga, Phymatolithon Foslie, inhibits the overgrowth of seaweeds without relying on herbivores. J Exp Mar Biol Ecol 96:127–146
Jones WC (1987) Skeletal variation in embryo-containing specimens of Haliclona rosea (Bowerbank) from Anglesey, North Wales. In: Vacelet J, Boury-Esnault N (eds) Taxonomy of Porifera. From the N.E. Atlantic and the Mediterranean Sea. Nato ASI Series G, Vol. 13, Springer, Berlin Heidelberg New York London Paris Tokyo, pp 101–124
Kinzie RA III (1973) Zonation of West Indian gorgonians. Bull Mar Sci 22:93–155
Laubenfels MW de (1949) The sponges of Woods Hole and adjacent waters. Bull Mus Comp Zool 103:1–55
Lauckner G (1980) Diseases of Porifera. In: Kinne O (ed) Diseases of marine animals, Vol I, General aspects, Protozoa to Gastropods. Wiley, Chichester, pp 139–165
Long ER (1968) The associates of four species of marine sponges of Oregon and Washington. Pacif Sci 22:347–351
Marszalek DJ, Gerehakov JM, Udey LR (1979) Influence of substrate composition on marine microfouling. App Environ Microbiol 38:987–995
McArthur DM, Moss BL (1977) The ultrastructure of cell walls in Enteromorpha intestinalis (L.) Link. Br Phycol J 12:359–368
Moss BL (1982) The control of epiphytes by Halydris siliquosa (L.) Lyngb. (Phaeophyta, Cystoseiraceae). Phycologia 21(2):185–191
O'Neill TB, Wilcox GL (1971) The formation of a “primary film” on materials submerged in the sea at Port Hueneme, California. Pac Sci 25:1–12
Patton WK (1972) Studies on the animal symbionts of the gorgonian coral Leptogorgia virgulata (Lamarck). Bull Mar Sci 22:413–419
Peattie ME, Hoare R (1981) The sublittoral ecology of the Menai Strait. II. The sponge Halichondria panicea (Pallas) and its associated fauna. Estuar Coast Shelf Sci 13:621–635
Russel G, Veltkamp CJ (1984) Epiphyte survival on skin-shedding macrophytes. Mar Ecol Progr Ser 18:149–153
Sarà M, Vacelet J (1973) Ecologie des démosponges. In: Grassé P-P (ed) Traité de Zoologie, Vol. III, part I, Spongiaires. Masson et Compagnie, Paris, p 481
Sieburth JMcN, Tootle JL (1981) Seasonality of microbial fouling on Ascophyllum nodosum (L.) Le Jol., Fucus vesiculosus L., Polysiphonia lanosa (L.) Tandy and Chondrus crispus Stackh. J Phycol 17:57–64
Silver MW, Gowing MM, Brownlee DC, Corliss JO (1984) Ciliated protozoa associated with oceanic sinking detritus. Nature 309:246–248
Thompson JE (1985) Exudation of biologically-active metabolites in the sponge Aplysina fistularis. I. Biological evidence. Mar Biol 88:23–26
Thompson JE, Walker RP, Faulkner DJ (1985) Screening and bioassays for biologically-active substances from forty marine sponge species from San Diego, California, USA. Mar Biol 88:11–21
Wahl M (1987) Epibiosis und Antifouling im Meer. Die Abwehrmechanismen der kolonialen Seescheide P. lacazei gegenüber dem Besiedlungsdruck durch potentielle Epibionten.Doctoral dissertation Universität Kiel, p 140
Walker RP, Thompson JE, Faulkner DJ (1985) Exudation of biologically-active metabolites in the sponge Aplysina fistularis. II. Chemical evidence. Mar Biol 88:27–32
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barthel, D., Wolfrath, B. Tissue sloughing in the sponge Halichondria panicea: a fouling organism prevents being fouled. Oecologia 78, 357–360 (1989). https://doi.org/10.1007/BF00379109
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00379109