Abstract
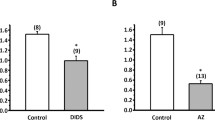
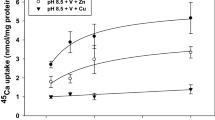
We examined transepithelial transport of Ca2+ across the isolated opercular epithelium of the euryhaline killifish adapted to fresh water. The opercular epithelium, mounted in vitro with saline on the serosal side and fresh water (0.1 mmol·l−1 Ca2+) bathing the mucosal side, actively transported Ca2+ in the uptake direction; net flux averaged 20–30 nmol·cm−2·h−1. The rate of Ca2+ uptake varied linearly with the density of mitochondria-rich cells in the preparations. Ca2+ uptake was saturable, apparent K 1/2 of 0.348 mmol·l−1, indicative of a multistep transcellular pathway. Ca2+ uptake was inhibited partially by apically added 0.1 mmol·l−1 La3+ and 1.0 mmol·l−1 Mg2+. Addition of dibutyryl-cyclic adenosine monophosphate (0.5 mmol·l−1)+0.1 mmol·l−1 3-isobutyl-l-methylxanthine inhibited Ca2+ uptake by 54%, but epinephrine, clonidine and isoproterenol were without effect. Agents that increase intracellular Ca2+, thapsigargin (1.0 μmol·l−1, serosal side), ionomycin (1.0 μmol·l−1, serosal side) and the calmodulin blocker trifluoperazine (50 μmol·l−1, mucosal side) all partially inhibited Ca2+ uptake. In contrast, apically added ionomycin increased mucosal to serosal unidirectional Ca2+ flux, indicating Ca2+ entry across the apical membrane is rate limiting in the transport. Verapamil (10–100 μmol·l−1, mucosal side), a Ca2+ channel blocker, had no effect. Results are consistent with a model of Ca2+ uptake by mitochondria rich cells that involves passive Ca2+ entry across the apical membrane via verapamil-insensitive Ca2+ channels, intracellular complexing of Ca2+ by calmodulin and basolateral exit via an active transport process. Increases in intracellular Ca2+ invoke a downregulation of transcellular Ca2+ transport, implicating Ca2+ as a homeostatic mediator of its own transport.
Similar content being viewed by others
Abbreviations
- DASPEI :
-
2-(4-dimethylaminostyryl)-N-ethylpyridinium iodide
- db-cAMP :
-
dibutyryl-cyclic adenosine monophosphate
- FW :
-
fresh water
- G t :
-
transepithelial conductance
- I sc :
-
short-circuit current
- IBMX :
-
3-isobutyl-1-methylxanthine
- SW :
-
sea water
- TFP :
-
trifluoperazine
- V t :
-
transepithelial potential
References
Cano TM, Perry SF, JC Fenwick (1994) Cholinergic control of stanniocalcin release in the rainbow trout, Oncorhynchus mykiss, and the American eel Anguilla rostrata. Gen Comp Endocrinol 94: 1–10
Clapham DE, Neher E (1984) Trifluoperazine reduces inward ionic currents and secretion by separate mechanisms in bovine chromaffin cells. J Physiol London 353: 544–564
Donald JA (1989) Adrenaline and branchial nerve stimulation inhibit 45Ca influx into the gills of rainbow trout, Salmo gairdneri. J Exp Biol 141: 441–445
Flik G, Wendelaar Bonga SE, Fenwick JC (1985) Active Ca2+ transport in plasma membranes of branchial epithelium of the north American eel, Anguilla rostrata LeSueur. Biol Cell 55: 265–272
Flik G, Schoenmakers TJM, Groot JA, Os CH van, Wendelaar Bonga SE (1990) Calcium absorption by fish intestine: the involvement of ATP- and sodium-dependent calcium extrusion mechanisms. J Membr Biol 113: 13–22
Flik G, Velden JA van der, Dechering KJ, Verbost PM, Schoenmakers TJM, Kolar ZI, Wendelaar Bonga SE (1993) Ca2+ and Mg2+ transport in gills and gut of tilapia, Oreochromis mossambicus: a review. J Exp Zool 265: 356–365
Foskett JK, Scheffey C (1982) The chloride cell: definitive identification as the salt-secretory cell in teleosts. Science 215: 164–166
Hogstrand C, Wilson RW, Polgar D, Wood CM (1994) Effects of zinc on the kinetics of branchial calcium uptake in freshwater rainbow trout during adaptation to waterborne zinc. J Exp Biol 186: 55–73
Kaneko T, Hirano T (1993) Role of prolactin and somatolactin in calcium regulation in fish. J Exp Biol 184: 31–45
Karnaky KJ Jr, Kinter LB, Kinter WB, Stirling CE (1976) Teleost chloride cell. II. Autoradiographic localization of gill Na+, K+-ATPase in killifish (Fundulus heteroclitus) adapted to low and high salinity environments. J Cell Biol 70; 157–177
Karnaky KJ Jr (1986) Structure and function of the chloride cell of Fundulus heteroclitus and other teleosts. Am Zool 26: 209–224
Lafeber FPJG, Flik G, Wendelaar Bonga SE, Perry SF (1988) Hypocalcin from Stannius corpuscles inhibits gill calcium uptake in trout. Am J Physiol 254: R891-R896
Liu C, Hermann TE (1978) Characterization of ionomycin as a calcium ionophore J Biol Chem 253: 5892–5894
Marshall WS (1977) Transepithelial potential and short-circuit current across the isolated skin of Gillichthys mirabilis (Teleostei: Gobiidae), acclimated to 5% and 100% seawater, J Comp Physiol 114: 157–165
Marshall WS (1986) Independent Na+ and Cl− active transport by urinary bladder epithelium of brook trout. Am J Physiol 250: R227-R234
Marshall WS, Bryson SE, Garg G (1993), 277-1 inhibition of Cl−1 transport by opercular epithelium is mediated by intracellular Ca2+. Proc Natl Acad Sci USA 90: 5504–5508
Marshall WS, Bryson SE, Wood CM (1992) Calcium transport by isolated skin of rainbow trout. J Exp Biol 166: 297–316
Marshall WS, Nishioka RS (1980) Relation of mitochondria-rich chloride cells to active chloride transport in the skin of a marine teleost. J Exp Zool 214: 147–156
Mashiko K, Jozuka K (1964) Absorption and excretion of calcium by teleost fishes with special reference to routes followed. Annot Zool Jpn 37: 41–50
Mayer-Gostan N, Bornancin M, DeRenzis G, Naon R, Yee JA, Shew RL, Pang PKT (1983) Extraintestinal calcium uptake in the killifish, Fundulus heteroclitus. J Exp Zool 227: 329–338
McCormick SD, Hasegawa S, Hirano T (1992) Calcium uptake in the skin of a freshwater teleost. Proc Natl Acad Sci USA 89: 3635–3638
Pang PKT, Griffith RW, Maetz J, Pic P (1980) Calcium uptake in fishes. In: Lahlou B (ed) Epithelial transport in the lower vertebrates. Cambridge University Press, Cambridge, pp 121–132
Payan P, Girard JP, Mayer-Gostan N (1984) Branchial ion movements in teleosts: the roles of respiratory and chloride cells. In: Hoar WS, Randall DJ (eds) Fish physiology, vol, 10B. Academic Press, New York, pp 39–63
Payan P, Mayer-Gostan N, Pang PKT (1981) Site of calcium uptake in the freshwater trout gill. J Exp Zool 216: 345–347
Péqueux A, Gilles R, Marshall WS (1988) NaCl transport in gills and related structures. In: Greger R (ed) Advances in comparative and Environmental physiology, vol I. Springer, Heidelberg pp 1–73
Perry SF, Flik G (1988) Characterization of branchial transepithelial calcium fluxes in the freshwater trout (Salmo gairdneri). Am J Physiol 254: R491-R498
Perry SF, Verbost PM, Vermette MG, Flik G (1988) Effects of epinephrine on branchial and renal calcium handling in the rainbow trout. J Exp Zool 246: 1–9
Perry SF, Wood CM (1985) Kinetics of branchial calcium uptake in the rainbow trout: effects of acclimation to various external calcium levels. J Exp Biol 116: 411–433
Philpott CW (1966) The use of horseradish peroxidase to demonstrate functional continuity between the plasmalemma and the unique tubular system of the chloride cell. J Cell Biol 31: 86A
Ussing HH (1949) The distinction by means of tracers between active transport and diffusion. Acta Physiol Scand 19: 43–56
Verbost PM, Flik G, Lock RAC, Wendelaar Bonga SE (1987) Cadmium inhibition of Ca2+ uptake in rainbow trout gills. Am J Physiol 253: R216-R221
Verbost PM, van Roolj J, Filk G, Lock RAC, Wendelaar Bonga SE (1989) The movement of cadmium through freshwater trout branchial epithelium and its interference with calcium transport. J Exp Biol 145: 185–197
Wood CM, Marshall WS (1994) Ion balance, acid-base regulation and chloride cell function in the common killifish Fundulus heteroclitus, a freely euryhaline estuarine teleost. Estuaries 17: 1A: 34–52
Author information
Authors and Affiliations
Additional information
Communicated by T. Hirano
Rights and permissions
About this article
Cite this article
Marshall, W.S., Bryson, S.E., Burghardt, J.S. et al. Ca2+ transport by opercular epithelium of the fresh water adapted euryhaline teleost, Fundulus heteroclitus . J Comp Physiol B 165, 268–277 (1995). https://doi.org/10.1007/BF00367310
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00367310