Summary
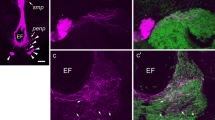
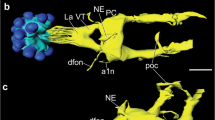
The pigment-dispersing hormone (PDH) family of neuropeptides comprises a series of closely related octadecapeptides, isolated from different species of crustaceans and insects, which can be demonstrated immunocytochemically in neurons in the central nervous system and optic lobes of some representatives of these groups (Rao and Riehm 1989). In this investigation we have extended these immunocytochemical studies to include the blowfly Phormia terraenovae and the cockroach Leucophaea maderae. In the former species tissue extracts were also tested in a bioassay: extracts of blowfly brains exhibited PDH-like biological activity, causing melanophore pigment dispersion in destalked (eyestalkless) specimens of the fiddler crab Uca pugilator. Using standard immunocytochemical techniques, we could demonstrate a small number of pigment-dispersing hormone-immunoreactive (PDH-IR) neurons innervating optic lobe neuropil in the blowfly and the cockroach. In the blowfly the cell bodies of these neurons are located at the anterior base of the medulla. At least eight PDH-IR cell bodies of two size classes can be distinguished: 4 larger and 4 smaller. Branching immunoreactive fibers invade three layers in the medulla neuropil, and one stratum distal and one proximal to the lamina synaptic layer. A few fibers can also be seen invading the basal lobula and the lobula plate. The fibers distal to the lamina appear to be derived from two of the large PDH-IR cell bodies which also send processes into the medulla. These neurons share many features in their laminamedulla morphology with the serotonin immunoreactive neurons LBO-5HT described earlier (see Nässel 1988). It could be demonstrated by immunocytochemical double labeling that the serotonin and PDH immunoreactivities are located in two separate sets of neurons. In the cockroach optic lobe PDH-IR processes were found to invade the lamina synaptic region and form a diffuse distribution in the medulla. The numerous cell bodies of the lamina-medulla cells in the cockroach are located basal to the lamina in two clusters. Additional PDH-IR cell bodies could be found at the anterior base of the medulla. The distribution and morphology of serotonin-immunoreactive neurons in the cockroach lamina was found to be very similar to the PDH-IR ones. It is hence tempting to speculate that in both species the PDH-and serotonin-immunoreactive neurons are functionally coupled with common follower neurons. These neurons may be candidates for regulating large numbers of units in the visual system. In the flies photoreceptor properties may be regulated by action of the two set of neurons at sites peripheral to the lamina synaptic layer, possibly by paracrine release of messengers.
Similar content being viewed by others
References
Barber RP, Sugihara H, Lee M, Vaughn JE, Salvaterra PM (1989) Localization of Drosophila neurons that contain choline acetyltransferase messenger RNA: an in situ hybridization study. J Comp Neurol 280:533–543
Bonomelli SL, Rao KR, Riehm JP (1989) Preparation and evaluation of an antiserum for crustacean α-PDH. Am Zool 29:49A
Boschek CB (1971) On the fine structure of the peripheral retina and lamina ganglionaris of the fly, Musca domestica. Z Zellforsch 118:369–409
Buchner E, Buchner S, Crawford G, Mason WT, Salvaterra PM, Sattelle DB (1986) Choline acetyltransferase-like immunoreactivity: in the brain of Drosophila melanogaster. Cell Tissue Res 246:57–62
Bülthoff H, Bülthoff I (1987a) GABA-antagonist inverts movement and object detection in flies. Brain Res 407:152–158
Bülthoff H, Bülthoff I (1987b) Combining neuropharmacology and behavior to study motion detection in flies. Biol Cybern 55:313–320
Datum K-H, Weiler R, Zettler F (1986) Immunocytochemical demonstration of γ-amino butyric acid and glutamic acid decarboxylase in R7 photoreceptors and C2 centrifugal fibers in the blowfly visual system. J Comp Physiol 159:241–249
Datum K-H, Rambolt I, Zettler F (1989) Cholinergic neurons in the lamina ganglionaris of the blowfly Calliphora erythrocephala. Cell Tissue Res 256:153–158
Dircksen H, Zahnow CA, Gaus G, Keller R, Rao KR, Riehm JP (1987) The ultrastructure of nerve endings containing pigment-dispersing hormone (PDH) in crustacean sinus glands: identification by an antiserum against synthetic PDH. Cell Tissue Res 250:377–387
Egelhaaf M, Borst A, Pilz B (1990) The role of GABA in detecting visual motion. Brain Res 500:156–160
Fernlund P (1976) Structure of a light-adapting hormone from the shrimp Pandalus borealis. Biochem Biophys Acta 439:17–25
Fingerman M, Rao KR, Bartell CK (1967) A proposed uniform method of reporting response values for crustacean chromatophorotropins: the Standard Integrated Response. Experentia 23:962
Gorczyca MG, Hall JC (1987) Immunohistochemical localization of choline acetyltransferase during development and in Chats mutants of Drosophila melanogaster. J Neurosci 7:1361–1369
Greenspan RJ (1980) Mutations of choline acetyltransferase and associated neural defects in Drosophila melanogaster. J Comp Physiol 137:83–92
Hardie R (1987) Is histamine a neurotransmitter in insect photoreceptors? J Comp Physiol 161:201–213
Hardie RC (1989a) A histamine-activated chloride channel involved in neurotransmission at a photoreceptor synapse. Nature 339:704–706
Hardie RC (1989b) Neurotransmitters in the compound eye. In: Stavenga DG, Hardie RC (eds). Facets of Vision. Springer, Berlin, pp 235–256
Hardie RC, Laughlin S, Osorio D (1989) Early visual processing in the compound eye: physiology and pharmacology of the retina-lamina projection of the fly. In: Singh RN, Strausfeld NJ (eds) Neurobiology of sensory systems. Plenum, New York, pp 23–42
Homberg U, Hildebrand JG (1989) Serotonin immunoreactivity in the optic lobes of the sphinx moth Manduca sexta and colocalization with FMRFamide and SCPB immunoreactivity. J Comp Neurol 288:243–253
Johansson O, Eckert M, Blechschmidt K (1990) Characterization of proctolinergic and GABAergic systems in the terminal ganglion of the male and female cockroach, Periplaneta americana, and effects of insecticide treatment. Immunohistochemistry and computer-assisted image analysis. J Chem Neuroanat 3:415–427
Lundquist CT, Rökaeus Å, Nässel DR (1991) Galanin immunoreactivity in the blowfly nervous system: localization and chromatographic analysis. J Comp Neurol (in press)
Mangerich S, Keller R (1988) Localization of pigment-dispersing hormone (PDH) immunoreactivity in the central nervous system of Carcinus maenas and Orconectes limosus (Crustacea) with reference to FMRFamide immunoreactivity in O. limosus. Cell Tissue Res 253:199–208
Mangerich S, Keller R, Dircksen H, Rao KR, Riehm JP (1988) Immunocytochemical localization of pigment-dispersing hormone and its coexistence with FMRFamide immunoreactive material in the eyestalks of the decapod crustaceans Carcinus maenas and Orconectes limosus. Cell Tissue Res 250:365–375
Meyer EP, Matute C, Streit P, Nässel DR (1986) Insect optic lobe neurons identifiable with monoclonal antibodies to GABA. Histochem 84:207–216
Morgan IG, Millar TJ, Ishimoto I, Boelen M, Dowton M, Chubb IW (1989) Functional studies on enkephalin-and somatostatin-immunoreactive amacrine cells in the chicken retina. In: Weiler R, Osborne NN (eds) Neurobiology of the inner retina. NATO ASI series. Springer, Berlin, pp 294–310
Nässel DR (1988) Serotonin and serotonin-immunoreactive neurons in the insect nervous system. Progr Neurobiol 30:1–85
Nässel DR (1989) Chemical neuroanatomy of the insect visual system. In: Singh RN, Strausfeld NJ (eds): Neurobiology of sensory systems. Pergamon Press, New York, pp 295–318
Nässel DR (1991) Neurotransmitters and neuromodulators in the insect visual system. Progr Neurobiol 37:179–254
Nässel DR, Klemm N (1983) Serotonin-like immunoreactivity in the optic lobes of three insects. Cell Tissue Res 232:129–140
Nässel DR, O'Shea M (1987) Proctolin-like immunoreactive neurons in the blowfly central nervous system. J Comp Neurol 265:437–454
Nässel DR, Meyer EP, Klemm N (1985) Mapping and ultrastructure of serotonin-immunoreactive neurons in the optic lobes of three insect species. J Comp Neurol 232:190–204
Nässel DR, Ohlsson L, Sivasubramanian P (1987) Postembryonic differentiation of serotonin-immunoreactive neurons in fleshfly optic lobes developing in situ or cultured in vitro without eye discs. J Comp Neurol 255:327–340
Nässel DR, Ohlsson LG, Johansson KUI, Grimmelikhuijzen CJP (1988a) Light and electron microscopic immunocytochemistry of neurons in the blowfly optic lobe reacting with antisera to RFamide and FMRFamide. Neuroscience 27:347–362
Nässel DR, Holmqvist MH, Hardie RC, Håkanson R, Sundler F (1988b) Histamine-like immunoreactivity in photoreceptors of the compound eyes and ocelli of the flies Calliphora erythrocephala and Musca domestica. Cell Tissue Res 253:639–646
Nicol D, Meinertzhagen IA (1982) An analysis of the number and composition of the synaptic populations formed by photoreceptors of the fly. J Comp Neurol 207:29–44
Pantin CFA (1934) The excitation of crustacean muscle. J Exp Biol 11:11–27
Rao KR, Riehm JP (1989) The pigment-dispersing hormone family: Chemistry, structure-activity relations, and distribution. Biol Bull 177:225–229
Rao KR, Mohrherr CJ, Riehm JP, Zahnow CA, Norton S, Johnson L, Tarr GE (1987) Primary structure of an analog of crustacean pigment-dispersing hormone from the lubber grasshopper Romalea microptera. J Biol Chem 262:2672–2675
Rao KR, Riehm JP, Zahnov CA, Kleinholz LH, Tarr GE, Johnson L, Norton S, Landau M, Semmes OJ, Sattelberg RM, Jorenby WH, Hintz MF (1985) Characterization of a pigment dispersing hormone in eyestalks of the fiddler crab Uca pugilator. Proc Natl Acad Sci USA 82:5319–5322
Rao KR, Mohrherr CJ, Bonomelli SL, Riehm JP, Kingan TG (1991) Insect neuropeptides: influence on color change in insects and chromatophoral pigment movements in crustaceans. In: Menn JJ, Kelly TJ, Masler (eds). Insect Neuropeptides: Chemistry, Biology and Actions, ACS Symposium Series 453. American Chemical Society, Washington, pp 110–122
Shaw SR (1984) Early visual processing in insects. J Exp Biol 112:225–251
Shaw SR, Fröhlich A, Meinertzhagen IA (1989) Direct connections between the R7/8 and R1-6 photoreceptor subsystems in the dipteran visual system. Cell Tissue Res 257:295–302
Strausfeld NJ (1971) The organization of the insect visual system (light microscopy). I. Projections and arrangements of neurons in the lamina ganglionaris of diptera. Z Zellforsch 121:377–441
Strausfeld NJ (1976) Atlas of an insect brain. Springer, Berlin
Strausfeld NJ (1989) Beneath the compound eye: Neuroanatomical analysis and physiological correlates in the study of insect vision. In: Stavenga D, Hardie RC (eds) Facets of Vision. Springer, Berlin, pp 317–358
Strausfeld NJ, Campos-Ortega JA (1977) Vision in insects: pathways possibly underlying neural adaptation and lateral inhibition. Science 195:894–897
Strausfeld NJ, Nässel DR (1980) Neuroarchitectures serving compound eyes of crustacea and insects. In: Autrum H (ed) Handbook of sensory physiology VII/6B. Springer, Berlin, pp 1–132
Würden S, Homberg U, Dirksen H, Rao KR (1990) Comparative morphology of PDH-immunoreactive neurons in the brain of orthopteroid insects. In: Elsner N, Roth G (eds) Brain, perception, cognition, Thieme, Stuttgart, p 316
Zahnow CA, Rao KR, Morherr CJ, Riehm JP (1987) Immunocytochemistry of neuropeptides in the cephalic neuroendocrine system of the lubber grasshopper, Romalea microptera. Soc Neurosci [Abstr 13], p 993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nässel, D.R., Shiga, S., Wikstrand, E.M. et al. Pigment-dispersing hormone-immunoreactive neurons and their relation to serotonergic neurons in the blowfly and cockroach visual system. Cell Tissue Res 266, 511–523 (1991). https://doi.org/10.1007/BF00318593
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318593