Summary
The distribution of serotonin immunoreactivity in the brain of the bullfrog (Rana catesbeiana) was studied, using the peroxidase-antiperoxidase (PAP) immunohistochemical method with serotonin antiserum.
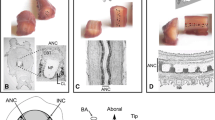
The somata of the serotonin neurons were mainly located in the raphe regions of the brain stem from the level of the caudal mesencephalon to that of the spinomedullary junction. A small number of serotonin neurons were also distributed as crebrospinal-fluid contacting neurons in the preoptic recess organ (PRO), the paraventricular organ (PVO), and the nucleus infundibularis dorsalis (Nid). In the raphe region, these serotonin neurons formed nearlycontinuous bilaterally-symmetrical cell columns along the caidline of the brain stem, divided into lateral and medial groups. The medial group was further subdivided into rostral and caudal parts. Processes of the serotonin neurons were widely distributed in the central nervous system, forming dense networks in various regions. The greates concentrations of these fibers were in the nucleus medialis speti, lateral portion of striatum, nucleus corporis geniculi, nucleus entopeduncularis, periventricular gray of ventral hypothalamus, optic tectum, nucleus isthmi, nucleus interpeduncularis, dorsal edge of medulla oblongata, and fasciculus solitarius.
Similar content being viewed by others
References
Aghajanian GK, Gallager DW (1975) Raphe origin of serotonergic nerves terminating in the cerebral ventricles. Brain Res 88:221–231
Ariëns Kappers CU, Huber C, Crosby EC (1960) The comparative anatomy of the nervous system of vertebrates including man. Vol. I, II and III Macmillan New York
Aronsson S, Enemar A (1981) On the structural and phyletic origin of the aminergic nerves of the hypophysis of frog tadpole (Rana temporaria) with special reference to pars distalis. J Comp Neurol 200:315–321
Bartels W (1971) Die Ontogenese der aminhaltigen Neuronsysteme im Gehirn von Rana temporaria. Z Zellforsch 116:94–118
Braak H (1970) Biogene Amine im Gehirn vom Frosch (Rana esculenta). Z Zellforsch 106:269–308
Chacko T, Terlou M, Peute J (1974) Fluorescence and electron microscopical study of aminergic nuclei in the brain of bufo poweri. Cell Tissue Res 149:481–495
Chan-Palay V (1976) Serotonin axons in the supra-and subependymal plexuses and in the leptomeninges; their roles in local alterations of cerebrospinal fluid and vasomotor activity. Brain Res 102:103–130
Dubé L, Parent A (1982) The organization of monoamine-containing neurons in the brain of the salamander, Necturus maculosus. J Comp Neurol 211:21–30
Goto M, Sano Y (1984) Ontogenesis of the central serotonin neuron system of the rat — an immunohistochemical study. Neuroscience Res 1:3–18
Hoffman HH (1963) The olfactory bulb, accessory olfactory bulb and hemisphere of some anurans. J Comp Neurol 120:317–368
Kawata M, Takeuchi Y, Ueda S, Masuura T, Sano Y (1984) Immunohistochemical demonstration of serotonin nerve fibers in the hypothalamus including posterior lobe of the rat and monkey. Cell Tissue Res (in press)
Kojima M, Takeuchi Y, Goto M, Sano Y (1982) Immunohistochemical study on the distribution of serotonin fibers in the spinal cord of the dog. Cell Tissue Res 226:477–491
Kondo Y, Nagatsu I, Yoshida M, Karasawa N, Nagatsu T (1983) Existence of noradrenalin cells and serotonin cells in the pituitary grand of Rana catesbeina. Cell Tissue Res 228:405–408
Lázár G (1978) Application of cobalt-filling technique to show retinal projections in the frog. Neuroscirice 3:725–736
Lázár GY, Székely GY (1969) Distribution of optic terminals in the different optic centers of the frog. Brain Res 16:1–14
Levitt P, Moore RY (1978) Developmental organization of raphé serotonin neuronal groups in the rat. Anat Embryol 154:241–251
Lidov HGW, Molliver ME (1982) Immunohistochemical study of the development of serotonergic neurons in the rat CNS. Brain Res Bull 9:559–604
Nakai Y, Ochiai H, Shioda S, Ochi J (1977) Cytological evidence for different types of cerebrospinal fluid-contacting subependymal cells in the preoptic and influndibular recesses of the frog. Cell Tissue Res 176:317–334
Neary T, Northeutt RG (1983) Nuclear organization of bullforg diencephalon. J Comp Neurol 213:262–278
Northcutt RG (1974) Some histochemical observations on the telencephalon of the bullfrog, Rana catesbeiana Shaw. J Comp Neurol 157:379–300
Opdam P, Kemali M, Nieuwenhuys R (1976) Topological analysis of the brain stem of the frogs Rana esculenta and Rana catesbeiana. J Comp Neurol 165:307–332
Parent A (1973) Distribution of monoamine-containing neurons in the brain stem of the frog, Rana temporaria. J Morphol 139:67–78
Parent A (1975) The monoaminergic innervation of the telencephalon of the forg, Rana pipiens. Brain Res 99:35–47
Potter HD (1969) Structural characteristics of cell and fiber populations in the optic tectum of the frog (Rana catesbeiana). J Comp Neurol 136:203–232
Prasada Rao PD (1982) Changes in formaldehyde-induced fluorescence of the hypothalamus and pars intermedia in the frog, Rana temporaria, following background adaptation. Brain Res Bull 9:765–776
Prasada Rao PD, Hartwig HG (1974) Monoaminergic tracts of the diencephalon and innervation of the pars intermedia in Rona temporaria. A fluorescence and microspectrofluorimetric study. Cell Tissue Res 151:1–26
Rámon Cajal P (1894) Investigaciones micrográficas en el encefalo de los batracios y reptiles, cuerpos geniculados y tuberculos cuadrigéminos de los mamíferos. La Dereche, Zaragoza
Richards JG, Lorenz HP, Tranzer JP (1973) Indolealkylamine nerve terminals in cerebral ventricles: identification by electron microscopy and fluorescence histochemistry. Brain Res 57:277–288
Sano Y, Ueda S, Yamada H, Takeuchi Y, Goto M, Kawata M (1983) Immunohistochemical demonstration of serotonin-containing CSF-contacting neurons in the submammalian paraventricular organ. Histochemistry 77:423–430
Scalia F (1976) The optic pathway of the frog: nuclear organization and connections. In: Llinás R, Precht W (eds) Frog neurobiology, Spinger, New York
Shimizu K, Kimura H, Ochi J (1983) Immunohistochemical demonstration of serotonin-containing subependymal cells in the frog hypothalamus. Histochemistry 79:23–29
Sims TJ (1977) The development of monoamine-containing neurons in the brain and spinal cord of the salamander, Ambystoma mexicanum. J Comp Neurol 173:319–336
Steinbusch HWM (1981) Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6:557–618
Steinbusch HWM, Verhofstad AAJ, Penke B, Varga J, Joosten HWJ (1981) Immunohistochemical charactarization of monoamine-containing neurons in the central nervous system by antibodies to serotonin and noradrenalin. A study in the rat and the lamprey (Lampetra fluviatilis). Acta Histochem Suppl XXIV:107–122
Takeuchi Y, Kimura H, Sano Y (1982) Immunohistochemical demonstration of the distribution of serotonin neurons in the brainstem of the rat and cat. Cell Tissue Res 224:247–267
Terlou M, Ploemacher RE (1973) The distribution of monoamines in the tel-, di- and mesencephalon of Xenopus laevis tadpoles, with special reference to the hypothalamo-hypophysial system. Z Zellforsch 137:521–540
Ueda S, Takeuchi Y, Sano Y (1983) Immunohistochemical demonstration of serotonin neurons in the central nervous system of the turtle (Clemmys japonica). Anat Embryol 168:1–19
Ueda S, Kawata M, Takeuchi Y, Sano Y (1983) Immunohistochemchemical demonstration of serotonin nerve fibers in the hypothalamus of the cat. Anat Embryol 168:315–330
Wada M, Urano A, Gorbman A (1980) A stereotaxic atlas for diencephalic nuclei of the forg, Rana pipiens. Arch Histol Jpn 43:157–173
Yamada H, Sano Y (in preparation) Immunohistochemical studies on the serotonin neuron system in the brain of the chicken (Gallus domesticus). II. The distribution of the nerve fibers
Yamada H, Takeuchi Y, Sano Y (1984) Immunohistochemical studies on the serotonin neuron system in the brain of the chicken (Gallus domesticus). I. The distribution of the neuronal somata. Biogenic Amines 1:17–28
Yoshida M, Nagatsu I, Kondo Y, Karasawa N, Ohno T, Spatz M, Nagatsu T (1983) Immunohistocytochemical localization of the neurons containing catecholamine-synthesizing enzymes and serotonin in the brain of bullfrog (Rana catesbiana). Acta Histochem Cytochem 16:245–258
Vigh-Teichmann I, Vigh B, Aros B (1968) Fluorescence histochemical studies of the preoptic recess organ in various vertebrates. Acta Biol Acad Sci Hung 20:423–436
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ueda, S., Nojyo, Y. & Sano, Y. Immunohistochemical demonstration of the serotonin neuron system in the central nervous system of the bullfrog, Rana catesbeiana . Anat Embryol 169, 219–229 (1984). https://doi.org/10.1007/BF00315627
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315627