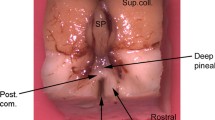
Summary
S-antigen-immunoreactive pinealocytes located in the deep portion of the pineal organ of inbred and wild pigmented mice give rise to long, beaded processes penetrating into the habenular and pretectal regions. In addition, the medial habenular nuclei and the pretectal area contain S-antigen-immunoreactive perikarya, which resemble pinealocytes in size, shape and immunoreactivity and are considered as “pinealocyte-like” epithalamic cells. Immunoblotting techniques reveal that a single protein band of approximately 48 kDa molecular weight accounts for this immunoreactivity. As shown with the use of the electron microscope, the majority of the S-antigen-immunoreactive processes is closely apposed to immunonegative neuronal profiles and perikarya of the habenular and pretectal regions. S-antigen-immunoreactive processes and perikarya of both pinealocytes of the deep pineal organ and pinealocyte-like epithalamic cells may form the postsynaptic element in conventional synapses involving axons provided with clear synaptic vesicles. Thus, certain mammalian pinealocytes may receive and transmit signals via point-to-point connections resembling neuro-neuronal contacts. These results challenge the concept that the mammalian pineal organ exerts its influence exclusively via the release of melatonin into the general circulation. Furthermore, they provide evidence (i) that neuronal circuits not involving the sympathetic system participate in the regulation of pineal functions in mammals, and (ii) that intimate histogenetic and functional relationships exist between the pineal organ and the habenular-pretectal nuclei in mammals.
Similar content being viewed by others
References
Bargman W (1943) Die Epiphysis cerebri. In: Möllendorff W von (ed) Handbuch der mikroskopischen Anatomie des Menschen. Vol. VI/4. Springer, Berlin Heidelberg, pp 309–502
Collin J-P (1979) Recent advances in pineal cytochemistry. Evidence of the production of indoleamines and proteinaceous substances by rudimentary photoreceptor cells and pinealocytes of Amniota. Prog Brain Res 52:271–296
Collin J-P (1981) New data and vistas on the mechanisms of secretion of proteins and indoles in the mammalian pinealocyte and its phylogenetic precursors; the pinealin hypothesis and preliminary comments on membrane traffic. In: Oksche A, Pévet P (eds) The pineal organ: photobiology-biochronometry-endocrinology. Elsevier, Asterdam, pp 187–210
Cozzi B, Møller M (1988) Indications for the presence of two populations of serotonin-containing pinealocytes in the pineal complex of the golden hamster (Mesocricetus auratus). Cell Tissue Res 252:115–122
Dowling JE, Dubin MW (1984) The vertebrate retina. In: Brookhart JM, Mounteastle VB (eds) Handbook of physiology. The Nervous System III, part 1. Am Physiol Soc, Bethesda, pp 317–339
Ekström P (1987) Photoreceptors and CSF-contacting neurons in the pineal organ of a teleost fish have direct axonal connections with the brain: An HRP-electron-microscopic study. J Neurosci 7:987–995
Ekström P, Foster RG, Korf HW, Schalken JJ (1987) Antibodies against retinal photoreceptor-specific proteins reveal axonal projections from the photosensory pineal organ in teleosts. J Comp Neurol 265:25–33
Hewing M (1978) A liquor contacting area in the pineal recess of the golden hamster (Mesocricetus auratus). Anat Embryol 153:295–304
Kalsow CM, Wacker WB (1973) Localization of a uveitogenic soluble retinal antigen in normal guinea pig eye by an indirect fluorescent antibody technique. Int Arch Allergy 44:11–20
Kalsow CM, Wacker WB (1977) Pineal reactivity of anti-retina sera. Invest Ophthalmol Visual Sci 16:181–184
Klein DC, Weller J, Sugden AL, Sugden D, Vanecek J, Chik CL, Ho AK (1987) Integration of multiple receptor mechanisms regulating pineal cyclic nucleotides. In: Trentini GP, Gaetani C de, Pévet P (eds) Fundamentals and clinics in pineal research. Raven Press, New York, pp 111–119
Korf HW, Møller M (1984) The innervation of the mammalian pineal gland with special reference to central pinealopetal projections. Pineal Res Rev 2:41–86
Korf HW, Møller M (1985) The central innervation of the mammalian pineal organ. In: Mess B, Ruszas C, Tima L, Pévet P (eds) The pineal gland. Current state of pineal research. Akad Kiado/Elsevier, Amsterdam, pp 47–69
Korf HW, Møller M, Gery I, Zigler JS, Klein DC (1983) Immunocytochemical demonstration of retinal S-antigen in the pineal organ of four mammalian species. Cell Tissue Res 239:81–85
Korf HW, Oksche A, Ekström P, Gery I, Zigler JS, Klein DC (1986a) Pinealocyte projections into the mammalian brain revealed with S-antigen antiserum. Science 231:735–737
Korf HW, Oksche A, Ekström P, van Veen T, Zigler JS, Gery I, Stein P, Klein DC (1986b) S-antigen immunocytochemistry. In: O'Brien P, Klein DC (eds) Pineal and retinal relationships. Academic Press, Orlando, pp 343–355
Korf HW, Sato T, Oksche A (1989) Reflections on the nature and connectivities of pincalocytes. In: Reiter RJ, Pang SF (eds) Advances in pineal research. Vol. 3. Libbey, London, pp 11–16
Kühn H (1984) Interactions between photoexcited rhodopsin and light-activated enzymes in rods. Prog Retinal Res 3:123–156
Lämmli UK (1970) Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature 227:680–685
Mirshahi M, Faure J-P, Brisson P, Falcon J, Gucrlotte J, Collin J-P (1984) S-antigen immunoreactivity in retinal rods and cones and pineal photosensitive cells. Biol Cell 52:195–198
Mirshahi M, Boucheix C, Collenot G, Thillaye B, Faure J-P (1985) Retinal S-antigen epitopes in vertebrate and invertebrate photoreceptors. Invest Ophthalmol Visual Sci 26:1016–1021
Møller M (1985) Non-sympathetic synaptic innervation of the pinealocyte of the Mongolian gerbil (Meriones unguiculatus). An electron microscopic study. J Neurocytol 14:541–550
Møller M, Korf HW (1987) Neural connections between the brain and the pineal gland of the golden hamster (Mesocricetus auratus). Cell Tissue Res 247:145–153
Müller B, Peichl L, Grip WJ de, Gery I, Korf HW (1989) Opsinand S-antigen-like immunoreactions in photoreceptors of the tree shrew retina. Invest Ophthalmol Visual Sci 30:530–535
Oksche A (1971) Sensory and glandular elements of the pineal organ. In: Wolstenholme GEW, Knight J (eds) The pineal gland. Churchill-Livingstone, Edinburgh, pp 127–146
Oksche A (1986) Historical perspectives of photoneuroendocrine systems. In: O'Brien P, Klein DC (eds) Pincal and retinal relationships. Academic Press, Orlando, pp 1–13
Oksche A (1990) Historical perspective: The development of the concept of photoneuroendocrine systems. In: Klein DC, Moore RY, Reppert SM (eds) Suprachiasmatic nucleus: The mind's clock. Oxford University Press, Oxford (in press)
Oksche A, Korf HW, Rodríguez EM (1987) Pinealocytes as photoneuroendocrine units of neuronal origin: Concepts and evidence. In: Reiter RJ, Fraschini F (eds) Advances in pineal research, Vol. 2. Libbey, London, pp 1–18
Parkington HC, McCane I, Coleman HA (1987) Two types of cells with central innervation in pineal glands of guinea pigs. Am J Physiol 252:C369–377
Pfister C, Chabre M, Plouet J, Tuyen VV, de Kosak Y, Faure J-P, Kühn H (1985) Retinal S-antigen identified as the 48K protein regulating light-dependent phosphodiesterase in rods. Science 228:891–893
Reuss S (1987) Electrical activity of the mammalian pineal gland. Pineal Res Rev 5:153–189
Rodríguez EM, Korf HW, Oksche A, Yulis CR, Hein S (1988) Pinealocytes immunoreactive with antisera against secretory glycoproteins of the subcommissural organ: A comparative study. Cell Tissue Res 254:469–480
Roussy G, Mosinger M (1938) Le complexe epithalamo-epiphysaire. Rev Neurol 69:459–470
Sakuranaga M, Naka KI (1985) Signal transmission in the catfish retina. I. Transmission in the outer retina. J Neurophysiol 53:373–389
Scharrer E (1964) Photo-neuro-endocrine systems: General concepts. Ann N Y Acad Sci 117:13–22
Steinbusch HWM (1984) Serotonin-immunoreactive neurons and their projections in the CNS. In: Björklund A, Hökfelt T, Kuhar MJ (eds) Handbook of chemical neuroanatomy, Vol. 3. Classical transmitters and transmitter receptors in the CNS, part II. Elsevier, Asterdam, pp 68–125
Vallerga S, Deplano S (1984) Differentiation, extent and lavering of amacrine cell dendrities in the retina of a sparid fish. Proc R Soc (Biol) 221:465–477
Veen T van, Elofsson R, Hartwig H-G, Gery I, Mochizuki M, Cena V, Klein DC (1986) Retinal S-antigen: Immunocytochemical and immunochemical studies on the distribution in animal photoreceptors and pineal organs. Exp Biol 45:15–25
Vigh-Teichmann I, Vigh B (1983) The sytem of cerebrospinal fluidcontacting neurons. Arch Histol Jpn 46:427–468
Vollrath L, Schröder H (1987) Neuronal properties of mammalian pinealocytes? In: Trentini GP, Gaetani C de, Pévet P (eds) Fundamentals and clinics in pineal research. Raven Press, New York, pp 13–23
Welsh M (1983) CSF-contacting pinealocytes in the pineal recess of the Mongolian gerbil: A correlative scanning and transmission electron microscope study. Am J Anat 166:483–493
Wiklund L (1974) Development of serotonin-containing cells and the sympathetic innervation of the habenular region in the rat brain. Cell Tissue Res 155:231–243
Wilden U, Wüst E, Weyand I, Kühn H (1986) Rapid affinity purification of retinal arrestin (48 K protein) via its light-dependent binding to phosphorylated rhodopsin. FEBS Lett 207:292–295
Yuwiler A (1983) Vasoactive intestinal peptide stimulation of pineal serotonin-N-acetyltransferase activity: General characteristics. J Neurochem 41:146–153
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Korf, HW., Sato, T. & Oksch, A. Complex relationships between the pineal organ and the medial habenular nucleus-pretectal region of the mouse as revealed by S-antigen immunocytochemistry. Cell Tissue Res 261, 493–500 (1990). https://doi.org/10.1007/BF00313528
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00313528