Summary
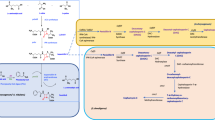
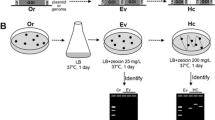
The cloning of five DNA segments carrying at least seven genes (fms1, fms3, fms4, fms5, fms7, fms11, and fms12) that participate in fortimicin A (astromicin) biosynthesis was described previously. These DNA fragments were used to screen a cosmid library of genomic DNA in order to examine if these biosynthetic genes are clustered in Micromonospora olivasterospora. One cosmid clone (pGLM990) was obtained, which hybridized to all the probes. Complementation analysis, using mutants blocked at various steps and chimeric plasmids subcloned from pGLM990, showed that three additional genes (fms8, fms10, and fms13) are present in pGLM990. A gene conferring self-resistance to the antibiotic, which was independently cloned in Streptomyces lividans, using the plasmid vector pIJ702 was also found to be linked to the cluster of biosynthetic genes. Thus, at least ten biosynthetic genes and a self-defense gene are clustered in a chromosomal region of about 27 kb in M. olivasterospora. Interestingly, the fms8 gene which participates in the dehydroxylation step of fortimicin A biosynthesis was found to have homology with a neomycin resistance gene nmrA from the neomycin-producing Micromonospora sp. MK50. Studies using a cell-free extract of the fms8 mutant and its parent strain showed that the enzyme encoded by fms8 phosphorylates a biosynthetic precursor, fortimicin KK1, in the presence of ATP. Thus the dehydroxylation reaction is suggested to occur via the phosphorylation of the target hydroxyl group. DNA regions homologous to fms genes were found in Micromonospora sp. SF-2098 and Dactylosporangium matsuzakiense, both producers of fortimicin group antibiotics.
Similar content being viewed by others
References
Baum EZ, Love SF, Rothstein DM (1988) Temporally regulated tandem promoters in Micromonospora echinospora. J Bacteriol 170:71–77
Baum EZ, Buttner MJ, Lin LS, Rothstein DM (1989) Transcription from the pl promoters of Micromonospora echinospora in the absence of native upstream DNA sequences. J Bacteriol 171:6503–6510
Chater KF (1990) The improving prospects for yield increase by genetic engineering in antibiotic producing Streptomyces Bio/Technology 8:115–121
Cortes J, Haydock SF, Roberts GA, Bevitt DJ, Leadlay PF (1990) An unusually large multifunctional polypeptide in the erythromycin-producing polyketide synthase of Saccharopolyspora erythraea. Nature 348:176–178
Dairi T, Hasegawa M (1989) Common biosynthetic features of fortimicin-group antibiotics. J Antibiot 42:934–943
Dairi T, Ohta T, Hashimoto E, Hasegawa M (1992a) Self cloning in Micromonospora olivasterospora of fms genes for fortimicin A (astromicin A) biosynthesis. Mol Gen Genet 232:262–270
Dairi T, Yamaguchi K, Hasegawa M (1992b) N-formimidoyl fortimicin A synthase, a unique oxidase involved in fortimicin A biosynthesis; Purification, characterization and gene-cloning. Mol Gen Genet, in press
Deushi T, Iwasaki A, Kamiya K, Mizoguchi T, Nakayama M, Itoh H, Mori T (1979) New aminoglycoside antibiotics, sannamycin. J Antibiot 32:1061–1065
Donadio S, Staver MJ, McAlpine JB, Swanson SJ, Katz L (1991) Modular organization of genes required for complex polyketide biosynthesis. Science 252:675–679
Goldberg SL, Romero JG, Deo YM (1990) Cloning and characterization of the sisomicin-resistance gene from Micromonospora inyoensis. J Antibiot 43:992–999
Hasegawa M, Dairi T, Ohta T, Hashimoto E (1991) A novel, highly efficient gene-cloning system for Micromonospora strains. J Bacteriol 173:7004–7011
Haydock SF, Dowson JA, Dhillon N, Roberts GA, Cortes J, Leadlay P (1991) Cloning and sequence analysis of genes involved in erythromycin biosynthesis in Saccharopolyspora erythraea. Mol Gen Genet 230:120–128
Hohn B, Collins J (1980) A small cosmid for efficient cloning of large DNA fragments. Gene 11:291–298
Hopwood DA, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser HM, Lydiate DJ, Smith CP, Ward JM, Schrempf H (1985) Gene manipulation of Streptomyces, a laboratory mannual. The John Innes Foundation, Norwich, UK
Itoh S, Odakura Y, Kase H, Satoh H, Takahashi K, Iida T, Shirahata K, Nakayama K (1984) Biosynthesis of astromicin and related antibiotics. J Antibiot 37:1664–1669
Iwasaki A, Ito H, Mori T (1979) A new broad spectrum aminoglycoside antibiotic complex, sporaricin. J Antibiot 32:180–186
Kelemen GH, Cundliffe E, Financsek I (1991) Cloning and characterization of gentamicin-resistance genes from Micromonospora purpurea and Micromonospora rosea. Gene 98:53–60
Kieser T (1984) Factors affecting the isolation of cccDNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19–36
Maehr H, Schaffner CP (1967) The separation and differentiation of the gentamicin complex. J Chromatogr 36:572–578
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Martin JF, Liras P (1989) Organization and expression of genes involved in the biosynthesis of antibiotics and other secondary metabolites. Annu Rev Microbiol 43:173–206
Nara T, Yamamoto M, Kawamoto I, Takayama K, Okachi R, Takasawa S, Sato T, Sato S (1977) Fortimicin A and B, new aminoglycoside antibiotics. I. Producing organism, fermentation and biological properties of fortimicins. J Antibiot 30:533–540
Odakura Y, Kase S, Itoh S, Satoh S, Takasawa S, Takahashi K, Shirahata K (1984) Biosynthesis of astromicin and related antibiotics. II. Biosynthetic studies with blocked mutants of Micromonospora olivasterospora. J Antibiot 37:1670–1680
Ohba K, Shomura T, Tsuruoka T, Kojima M, Inoue S, Itoh T (1981) Production of antibiotics SF-2050. Patent, JP 18600, Feb 21, 1981
Ohta T, Nagano E, Dairi T, Hasegawa M (1988a) In: 7th International Symposium on Biology of Actinomycetes, Japan, May 22–26, P3–28
Ohta T, Nagano E, Dairi T, Hasegawa M (1988b) In: 7th International Symposium on Biology of 4ctinomycetes, Japan, May 22–26, P3–29
Okachi R, Kawamoto I, Takasawa S, Yamamoto M, Sato S, Sato T, Nara T (1974) A new antibiotic XK-62-2(sagamicin). I. Isolation, physicochemical and antibacterial properties. J Antibiot 27:793–800
Okami Y, Hotta K, Yoshida M, Ikeda D, Kondo S, Umezawa H (1979) New aminoglycoside antibiotics istamycin A and B. J Antibiot 32:964–966
Shomura T, Kojima M, Yoshida J, Ito M, Amano S, Totsugawa K, Niwa T, Inouye S, Ito T, Niida T (1980) Studies on a new aminoglycoside antibiotic, dactimicin. J Antibiot 33:924–930
Stanzak R, Matsushima P, Baltz RH, Rao RN (1986) Cloning and expression in Streptomyces lividans of clustered erythromycin biosynthesis genes from Streptomyces erythreus. Bio/Technology 4:229–232
Thompson CJ, Kieser T, Ward JM, Hopwood DA (1982a) Physical analysis of antibiotic-resistance genes from Streptomyces and their use in vector construction. Gene 20:51–62
Thompson CJ, Skinner RH, Thompson J, Ward JM, Hopwood DA, Cundliffe E (1982b) Biochemical characterization of resistance determinants cloned from antibiotic-producing Streptomyces. J Bacteriol 151:678–685
Thompson CJ, Skeggs PA, Cundliffe E (1985) Methylation of 16S ribosomal RNA and resistance to the aminoglycoside antibiotics gentamicin and kanamycin determined by DNA from the gentamicin-producer, Micromonospora purpurea. Mol Gen Genet 201:168–173
Weber KM, Leung JO, Maine GT, Potenz RHB, Paulus TJ, DeWitt JP (1990) Organization of cluster of erythromycin genes in Saccharopolyspora erythraea. J Bacteriol 172:2372–2383
Weinstein MJ, Marquez JA, Testa RT, Wagman GH, Oden EM, Waitz JA (1970) Antibiotic 6640, a new Micromonospora produced aminoglycoside antibiotic. J Antibiot 23:551–554
Author information
Authors and Affiliations
Additional information
Communicated by H. Hennecke
Rights and permissions
About this article
Cite this article
Dairi, T., Ohta, T., Hashimoto, E. et al. Organization and nature of fortimicin A (astromicin) biosynthetic genes studied using a cosmid library of Micromonospora olivasterospora DNA. Molec. Gen. Genet. 236, 39–48 (1992). https://doi.org/10.1007/BF00279641
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00279641