Abstract
Horizontal gene transfer occurs frequently in bacteria, but the mechanism driving activation and optimization of the expression of horizontally transferred genes (HTGs) in new recipient strains is not clear. Our previous study found that spontaneous tandem DNA duplication resulted in rapid activation of HTGs. Here, we took advantage of this finding to develop a novel technique for tandem gene duplication, named tandem gene duplication selected by activation of horizontally transferred gene in bacteria (TDAH), in which tandem duplication was selected by the activation of horizontally transferred selectable marker gene. TDAH construction does not contain any reported functional elements based on homologous or site-specific recombination and DNA amplification. TDAH only contains an essential selectable marker for copy number selection and 9-bp-microhomology border sequences for precise illegitimate recombination. One transformation and 3 days were enough to produce a high-copy strain, so its procedure is simple and fast. Without subsequent knockout of the endogenous recombination system, TDAH could also generate the relatively stable high-copy tandem duplication for plasmid-carried and genome-integrated DNA. TDAH also showed an excellent capacity for increase gene expression and worked well in different industrial bacteria. We also applied TDAH to select the optimal high copy number of ribA for vitamin B2 production in E. coli; the yield was improved by 3.5 times and remained stable even after 12 subcultures. TDAH is a useful tool for recombinant protein production and expression optimization of biosynthetic pathways.
Key points
• We develop a novel and efficient technique (TDAH) for tandem gene duplication in bacterium. TDAH is based on the mechanism of HTG rapid activation. TDAH does not contain any reported functional elements based on homologous recombination and DNA amplification. TDAH only contains an essential selectable marker for copy number selection, so its construction and procedure are very simple and fast.
• TDAH is the first reported selected and stable tandem-gene-duplication technique in which the selected high-copy plasmid-carried and genome-integrated DNA could remain stable without the subsequent knockout of recombination system.
• TDAH showed an excellent capacity for regulating gene expression and worked well in different industrial bacteria, indicating it is a useful tool for recombinant protein production and expression optimization of biosynthetic pathways.
• TDAH was applied to select the optimal high copy number of ribA for vitamin B2 production in E. coli; the yield was improved by 3.5-fold and remained stable even after 12 subcultures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Horizontal gene transfer (HGT), also known as lateral gene transfer (LGT), is the exchange of genetic information between unrelated organisms, which stands in contrast to vertical gene transfer (VGT) from mother to daughter cell. HGT has occurred frequently in all domains of life, especially in prokaryotes (such as bacteria), and contributed substantially to prokaryotic genome evolution (Lan and Reeves 1996; Ochman et al. 2000; Gogarten et al. 2002; Boucher et al. 2003; Dagan et al. 2008; Brockhurst et al. 2011; Ginty et al. 2011; Popa et al. 2011; Treangen and Rocha 2011; Bock 2017). However, the molecular mechanisms driving these HGT evolutionary events are still not fully understood. It is conceivable that in addition to the uptake and integration of foreign DNA, the proper expression of exogenous genes is also necessary for functional HGT events, which confer a selective advantage to the recipient, leading to the retention of horizontally transferred genes during evolution. However, the gene expression systems of different species are generally incompatible, and the expression elements will not function normally in a new recipient, so the freshly horizontally transferred genes are mostly silent (Thomas and Nielsen 2005; Popa and Dagan 2011). These foreign genes must be activated spontaneously and rapidly before they are lost during evolution. Promoter capture and gene duplication has been suggested to represent a major mechanism driving the activation and optimization of previously silent genes (Stegemann and Bock 2006; Matus-Garcia et al. 2012; Elliott et al. 2013; Katju and Bergthorsson 2013; Oren et al. 2014), such as the activation of citrate transporter gene in the famous E. coli long-term evolution experiment (Blount et al. 2012). Promoter capture is usually caused by fusion of silent gene coding region and adjacent promoter, which is mediated by DNA deletion or tandem duplication, such as the activation of histidine genes in Salmonella typhimurium (Anderson and Roth 1978). Our previous experimental evolution study showed that spontaneous promoter capture events prior to selection lead to rapid activation of horizontally transferred genes in E.coli (Li and Bock 2019). Some promoter capture events were also attributed to the evolutionary events of tandem DNA duplication, which were not mediated by classical homologous regions, and do not need any homologous sequences (illegitimate recombination). Therefore, we found that spontaneous and quick tandem DNA duplication events prior to selection pressure resulted in promoter capture, leading in turn to the rapid activation of horizontally transferred gene. Thus, rapid functional activation of a horizontally transferred gene may be mediated by quick spontaneous tandem DNA duplication. However, the regulatory mechanisms underlying this type of tandem duplication were still not clear, because this construction did not contain any reported functional elements of homologous recombination and DNA amplification. In this study, we took advantage of the aforementioned HTG activation by tandem duplication to develop a novel and simple technique for bacterial multi-copy tandem gene duplication, named tandem gene duplication selected by activation of horizontally transferred gene (TDAH), in which the tandem duplication is selected by the activation of horizontally transferred selectable marker gene.
Tandem gene duplication is a focus of research on genome evolution and is widely used in gene expression regulation and biosynthetic pathway optimization. Accordingly, a powerful technique for tandem gene duplication is very important for basic and synthetic biology. At present, there are two strategies for multi-copy tandem gene duplication, based either on in vitro or in vivo construction. In vitro methods mainly include the isocaudamer method, asymmetric sticky end complementary ligation, PCR tandem amplification, and directional adapter method (Li et al. 2003; Rao et al. 2005; Tian et al. 2007; Wu et al. 2010).Most of the existing in vitro methods used PCR amplification systems, combined with DNA restriction endonucleases and DNA ligase to artificially add repeating units and gradually increase the copy number, so that subsequent multiple transformations generate E. coli transformants with different copy numbers. However, these in vitro methods are often time-consuming and it is sometimes difficult to select suitable restriction enzymes, and excessive-copy tandem DNA is usually not stable in vitro. An in vivo strategy could solve the problems of in vitro methods and showed the tremendous potential for the applications in gene expression regulation and biosynthetic pathway optimization. Several in vivo methods have been reported, such as Cre/loxP-mediated multi-copy integration, amplification of a haploinsufficient gene (HapAmp), chemically inducible chromosomal evolution (CIChE), and evolution by amplification and synthetic biology (EASy) (Tyo et al. 2009; Yin et al. 2015; Peng et al. 2022; Pardo et al. 2023). In these in vivo methods, the copy numbers were selected based on the dosage of a fitness-associated gene (antibiotic resistance gene) in the repeating unit, but the mechanisms of DNA recombination and amplification were not the same and the required elements include DNA recombinases (Cre, RecA, etc.), specific DNA sequence site (loxP), autonomous replicating sequence (ARS), and the homologous regions, so the exogenous functional elements need to be simultaneously or beforehand introduced into the host cell; for example, EASy is mediated by synthetic DNA bridging fragment (SBF), which is an approximate 2000-bp synthetic homologous sequence and is individually transformed into host cell for homologous-recombination-mediated gene duplication. At the same time, to make the high copy number stable, the endogenous recombination system usually needs to be deleted after gene amplification to prevent subsequent homologous recombination that causes the copy number to decline. In this study, we developed a simple, stable technique for tandem gene duplication based on our previous finding that spontaneous tandem DNA duplication resulted in rapid HTG activation. TDAH construction only contains an essential selectable marker gene split at the flanks of the gene of interest, resulting in a very streamlined protocol. Moreover, TDAH worked well for both tandem DNA duplication on the E. coli chromosome or on a plasmid, and the copy number could be relatively stable needless of subsequent recA knockout. In addition to Gram-negative E. coli, TDAH also worked in Gram-positive Bacillus subtilis, indicating that it may be widely applicable in different bacteria.
As an essential micronutrient for human and animal growth and reproduction, vitamin B2 (riboflavin) plays crucial roles in the synthesis of flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN), as well as in the conversion of tryptophan into niacin (Averianova et al. 2020). However, the human body’s capacity for storing vitamin B2 is limited, leading to a relatively common occurrence of latent riboflavin deficiency, particularly among women and adolescents in developing countries (Bacher et al. 2000). Recent advancements in microbial cell factory technology have shown promise in enhancing vitamin B2 production. As reported, proper and reasonable expression of ribA was regarded as a key factor for vitamin B2 production (Lin et al.2014). We applied TDAH to select the optimal high copy number of ribA for vitamin B2 production in E. coli.
Materials and methods
Construction of TDAH starting strains
The schematic diagram of TDAH is shown in Fig. 1. One repeating unit as described in Fig. 2A was synthesized by GENEWIZ company (https://www.genewiz.com.cn) and was inserted into the vector backbone of pUC57, resulting in the pOr1 plasmid. The repeating unit of TDAH was made up of the lower half of the ble cassette (Lb), the gene of interest (GOI), and the upper half of the ble cassette (Ub). In pOr1, ble expression was driven by the weak promoter J23116 from the Registry of Standard Biological Parts (http://parts.igem.org/Part:BBa_J23119). The J23116 promoter was located in the Ub, and Lb contained the ble coding sequence and J23116 terminator. The GOI of pOr1 was a GFP gene driven by the weak araBAD promoter (Zhao et al. 2017). The original strain (Or) was constructed by introducing the pOr1 plasmid into E. coli strains K-12 MG1655 and DH5ɑ, respectively named as Or1 and Or4 starting strains. The pOr1 plasmid was introduced into E. coli strains K-12 MG1655 and DH5ɑ, respectively resulting in the Or1 and Or4 starting strains for TDAH. Based on the repeating unit of Or1, the ble expression system was changed to Prrn (Li and Bock 2019), and the resulting repeating unit together with a kanamycin resistance gene was inserted into the lac operon locus in the genome of MG1655 by λRed recombination to form the Or2 starting strain (Fig. 3A). Table S1 shows the 150-bp homologous sequences flanking the lac locus. The pOr3 vector was constructed for TDAH of plasmid DNA in Bacillus subtilis, and ble expression was driven by the sp110 promoter (Liu et al. 2018). The GOI of pOr3 was mCherry, and the resulting repeating unit was inserted into the vector backbone of pHP13 to form the pOr3 plasmid. Then, pOr3 was introduced into Bacillus subtilis strain Bs168, resulting in the Or3 starting strain for TDAH. The whole open reading frame of recA gene was deleted by λRed recombination, and repeating unit was introduced into lac operon locus (Or2) to form Or5. Based on the plasmid of pOr1, with the GFP gene replaced by ribA and driven by an artificial moderately strong promoter, PWGAN-1–4 (Wang et al.2020), resulting in the pOr6 plasmid, the pOr6 plasmid was introduced into E. coli strains K-12 MG1655 to form the Or6 starting strain. All primers are shown in Table S1.
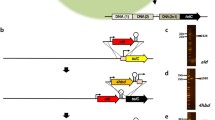
Schematic diagram of tandem gene duplication selected by activation of horizontally transferred gene (TDAH). A Overview of the molecular rearrangements for TDAH evolution events. In the original strain (Or), the antibiotic resistance marker ble expression cassette on the plasmid or the genome was split in half, the upper half of the ble cassette (Ub) contained promoter and was placed downstream of the gene of interest (GOI), while the lower half of the ble cassette (Lb) was placed upstream, so ble was inactive in Or. In the evolved strain (Ev), the TDAH evolution event resulted in the reconstitution of a functional ble expression cassette (Cb), which could be selected using antibiotics. The high-copy strain (Hc) could be selected by increasing the antibiotic concentration. B Experimental protocol of TDAH. The Ev strains were selected from the Or culture using by 25 mg/L zeocin, and Hc strains were selected by streaking Ev onto a plate with 200 mg/L zeocin. Ev and Hc were identified by PCR or restriction analysis
Selected and stable multi-copy tandem duplication of E.coli plasmid DNA using the TDAH technique and its application in gene expression regulation. A Schematic map of TDAH vector pOr1 and its tandem-duplication evolved plasmid (Ev1). The total length of the pOr1 vector was 4.3 kb. The length of the repeating unit was 1.6 kb, and the restriction sites (KpnI and AscI) are located outside of the repeating unit. SmaI cuts pOr1 only once (in the Lb region). Arrows indicate the primers (ble-rp and ble-fp). One 0.5-kb (0.4 + 0.1) PCR fragment obtained using the above primers indicated that the complete ble cassette (Cb) was reconstituted, and tandem duplication occurred. The ble gene promoter (Pb1) and its matching terminator (Tb1) are derived from the weak expression system J23116. Microhomology border sequences (MHS) were added flanking the repeating unit. Complete ble cassette in Ev1 showed the sequence information of MHS and join point; the enlarged ATG indicated the initiation codon. B Identification of the tandem-duplication evolved strain (Ev1). Ev1 was selected by zeocin and identified by PCR with ble-fp and ble-rp (one 0.5-kb fragment in the left electrophoretogram). To confirm the expected recombination of plasmid sequence in Ev1, Ev1 plasmids were cut with KpnI and AscI. In the original strain (Or), pOr1 was cut into two fragments, the backbone (2.7 kb) and one repeating unit (1.6 kb), while in Ev1, a tandem-duplication fragment appeared (1.6 × 2 = 3.2 kb in the right electrophoretogram). There was still the one-repeating-unit fragment in some Ev1 strains, indicating tandem-duplication plasmid has not been completely homogenized. C Identification of the high-copy strain (Hc1). The high-copy strain could be selected by higher concentrations of zeocin and was identified by the total length of the tandem-duplication region (fragment flanked by KpnI and AscI restriction sites). The left electrophoretogram shows that most high-copy strains (Hc1) have long restriction fragments (> 15 kb). To confirm the expected recombination of the tandem-duplication region (long restricted fragments) in Hc1, the plasmids were digested with SmaI, which cut once in the repeating unit, as shown in the right electrophoretogram. D Stability of tandem-duplication copy number of Hc1 during continuous subculture. High-copy strain Hc1-3 was subcultured in liquid LB medium for five consecutive rounds with or without antibiotic selection pressure (zeocin: 0 mg/L, 25 mg/L, and 200 mg/L). The plasmids from 1st, 3rd, and 5th subcultures were cut with KpnI and AscI. E Correlation between the tandem-duplication copy number and the expression of the gene of interest (GFP) in TDAH strains. The copy number was calculated based on the size of the tandem-duplication fragment (KpnI and AscI). The fluorescence intensity analysis indicated that the expression of the gene of interest was enhanced as the tandem-duplication copy number increased in TDAH strains
Selected and stable multi-copy tandem duplication of genome-integrated DNA in E. coli using the TDAH technique. A Schematic map of the repeating unit in the lac operon locus in the genome of the original strain (Or2). The length of the repeating unit was 1.6 kb. Arrows indicate primers. B Identification of the tandem-duplication evolved strain (Ev2). Ev2 could be selected by zeocin and identified by PCR with ble-fp and ble-rp [one 0.7 kb (0.5 + 0.2) fragment]. C Identification of the high-copy strain (Hc2). Hc2 could be selected by a higher concentration of zeocin. The tandem-duplication copy numbers were measured by qPCR of ble on genomic DNA normalized to Or2. The genomic DNA of the Hc2 strains was digested with SmaI and HindIII, and the Southern blot (right picture) was hybridized with a ble probe. D Stability of copy number and expression of the gene of interest in Hc2-6. Strain Hc2-6 with the highest copy number was subcultured for five consecutive rounds with or without antibiotic selection pressure (zeocin: 0 mg/L and 25 mg/L). The copy numbers were measured in the 1st, 3rd, and 5th subcultures. We did not find the obvious changes in the copy number with or without zeocin in the left histogram, indicating that the copy number remained stable during continuous subculture. As shown in the right histogram, quantification of GFP mRNA accumulation by qRT-PCR indicated that the high-copy strain had higher expression of the gene of interest
Selection and identification of tandem duplication in the TDAH process
To select evolved strain (Ev), cultures comprising 30-mL liquid Luria–Bertani (LB) medium were inoculated with a single colony of each Or strain and cultivated at 37 °C on a rotary shaker at 180 rpm for 1 day. Aliquots of the resulting bacterial culture were spread on agar plates (15 cm diameter) with LB medium containing 25 mg/L zeocin and incubated at 37 °C overnight to identify resistant colonies (Ev strains). The liquid culture of each Or strain could be expanded to increase the cell number of starting strain and obtain more Ev strains. High-copy strain (Hc) was selected by streaking Ev on LB plates with 200 mg/L zeocin. Ev and Hc were identified by PCR or restriction analysis using the PCR primers shown in Table S1 and restriction enzymes indicated in the text and figures, respectively.
Restriction analysis, Southern blot, real-time quantitative PCR (qPCR), real-time quantitative reverse-transcription PCR (qRT-PCR), and fluorescence analysis
Plasmids were extracted from bacterial cultures using the TIANprep Mini Plasmid Kit (Tiangen Biotech, Beijing). The restriction enzymes included KpnI and AscI (New England Biolabs) and SmaI and HindIII (Thermo Fisher Scientific). The results of restriction were analyzed by 1% agarose gel electrophoresis.
For southern blot analysis, DNA was extracted from bacterial cultures using the TIANamp Bacterial DNA Kit (Tiangen Biotech, Beijing). Restricted bacterial DNA was separated by 1% agarose gel electrophoresis and transferred to an NX nylon membrane (GE Healthcare) by capillary blotting. For preparation of hybridization probes, the ble coding sequence was amplified by PCR using specific primers (Table S1), and this probe was labeled with digoxin using the PCR DIG Probe Synthesis Kit (Roche, Indianapolis, IN, USA). Hybridization was performed at 47 °C for 16 h using standard protocols. Then, the membrane was washed, exposed with chemiluminescence reagent, and recorded in a luminescence imager (Tanon 5200 Multi, China).
For qRT-PCR analysis, RNA from E. coli cultures was extracted using the RNA prep Pure Cell Bacteria Kit (Tiangen Biotech, Beijing), and cDNA was synthesized using M-MLV Reverse Transcriptase (Promega Biotech, Beijing), Recombinant RNasin® Ribonuclease Inhibitor (Promega Biotech, Beijing), and dNTP Mixture (Biomed, Beijing). Subsequent amplification was performed using the ChamQ Universal SYBR QPCR Master Mix (Vazyme Biotech, China) on a Real-Time PCR System (ABI 7500 FAST, Applied Biosystems, USA). The GFP mRNA level was quantified relative to the endogenous GAPDH gene. Each experiment had three independent biological replicates, and the 2−∆∆CT (cycle threshold) method was used to determine relative cDNA levels. Microsoft Office Excel 2010 and IBM SPSS Statistics 19 were used to analyze the experimental data. Error bars indicate the standard error for the sample mean. All primers are listed in Table S1. For the qPCR experiment, strains of Or, Ev, and Hc were used as templates, using bioA (in Hc2 and Hc4) and Spec (in Hc3) as reference genes and ble as target genes. Then, analysis of copies was performed based on reference genes and target genes.
The flow cytometry instrument MoFlo™ XDP (Beckman Coulter Inc. Brea, CA, USA) was used for fluorescence analysis of strains with different copy numbers. The mean value of fluorescence detected by flow cytometry was used as an index of the expression level of each strain. The cells were diluted in PBS, and GFP fluorescence was measured with the green (488 nm) laser and 529-nm filter. The forward scatter (FSC) voltage and GFP voltage were set to 150 and 450. For each sample, 100,000 events were recorded. The results were analyzed using MoFlo XDP Summit 5.2 software (Beckman Coulter Inc., Brea, CA, USA).
The concentration of vitamin B2 was analyzed with the wavelengths of λex 473 nm and λem 520 nm, respectively (Chen et al. 2006).
Copy number stability assay of the Hc strain
The assay was based on five successive subcultures of Hc at a 1:100 dilution in LB medium with or without antibiotic (zeocin: 0 mg/L, 25 mg/L, and 100 mg/L). The 1st, 3rd, and 5th subcultures were sampled, and the extracted plasmids were identified by restriction analysis (KpnI and AscI).
Results
Selected and stable multi-copy tandem duplication of E. coli plasmid DNA using the TDAH technique and its application in gene expression regulation
Our previous experimental evolution study found that spontaneous and quick tandem DNA duplication events (illegitimate recombination) resulted in promoter capture, leading to the rapid activation of horizontally transferred genes. In this study, we wanted to take advantage of this finding to develop a novel technique for bacterial multi-copy tandem DNA duplication, named tandem gene duplication selected by activation of horizontally transferred gene (TDAH), in which the tandem duplication is selected by the activation of a horizontally transferred selectable marker gene. Different from the existing in vivo tandem duplication methods, there are no reported functional elements of tandem duplication or homologous recombination in this TDAH system. Instead, the TDAH system only relies on the obscure evolutionary mechanism for rapid activation of horizontally transferred genes, only containing an essential selectable marker for copy number selection and only requiring the addition of this split marker gene flanking the gene of interest. This results in a very streamlined protocol, as shown in Fig. 1. We just needed to split an antibiotic resistance gene (ble expression cassette) in half and place Ub (containing the promoter) downstream of GOI, as well as Lb upstream, resulting in one repeating unit. Although our previous experimental evolution study (Li and Bock 2019) showed that the microhomology sequences were not necessary for duplication mutation. In order to induce our expected precise duplication mutation, we added the microhomology border sequences (GC rich, similar as the microhomology sequences found in previous study (Li and Bock 2019)) flanking the repeating unit to form our designed join point (Fig. 2A). Then, this repeating unit was introduced into the bacterial host to form the Or for TDAH. Initially, the ble cassette was inactive (no expression) in Or. If the target tandem-duplication event occurred, the resulting complete ble expression cassette (Cb) leads to functional activation of ble in the Ev, which can be selected on zeocin plates. In order not to introduce the foreign vector sequence into the Cb of Ev strains, we added a stop codon (TGA) upstream of Lb, avoiding the selection of strains in which the upstream vector sequence was duplicated together with Lb. A Hc could be generated and selected by increasing the antibiotic concentration.
To develop a TDAH protocol for E. coli plasmid sequences, we constructed the pOr1 vector (Fig. 2A), carrying a repeating unit consisting of Lb, gene of interest (GFP), and Ub. To be able to detect the dosage effect of the ble cassette, the promoter of ble (Pb1) and its matching terminator (Tb1) were based on the weak expression system J23116 from the Registry of Standard Biological Parts. The GOI of pOr1 was a GFP gene driven by the weak araBAD promoter (Zhao et al. 2017). The pOr1 vector was introduced into wild-type E. coli MG1655 to construct Or1 for TDAH. Overnight cultures of Or1 were selected on LB agar plates containing 25 mg/L zeocin, and ble-activated evolved strains Ev1 (resistant colonies) were further identified by PCR with primers specific for the complete ble expression cassette. The restriction analysis of Ev1 plasmids also confirmed the expected recombination of the evolved plasmid (Fig. 2B). The formation of Cb was also confirmed by sequencing of PCR products; even though several bp of the vector sequence was occasionally introduced between Ub and Lb in a minority of Ev strains (not affecting the expression of ble), almost all of Ev strains had our designed and precise join point. By counting the number of Ev colonies and the total cell number in the Or culture, we could also calculate the mutant frequency of Ev1 (colony number of Ev/total cell number of the Or culture), and it was approximately 3.6 × 10−11, which meant that approximately 1.6 Ev1 colonies could be selected from 100 mL of Or1 culture (Table 1).
Due to the dosage effect, the high-copy strain Hc1 could be selected by increasing the zeocin concentration to 200 mg/L. The resulting Hc1 colonies were further identified by the total length of the tandem-duplication region. Restriction analysis showed that most high-copy strains (Hc1) had long restricted fragments (> 15 kb), which indicated high tandem-duplication copy numbers of more than 10. To confirm the expected recombination of the tandem-duplication region (long restricted fragments) in Hc1, the plasmids were restricted with an endonuclease that cuts once in the repeating unit. The results showed that the tandem-duplication regions (long restricted fragments) contained only the repeating units with different copy numbers (Fig. 2C). Almost all colonies that grew on plates with 200 mg/L had more than two copies of the repeating unit (multi-copy). Accordingly, 3 days was sufficient to evolve the high-copy tandem DNA duplication strain from the original single-copy strain.
To test the stability of tandem-duplication copy number in Hc1, the high-copy (10 +) strain Hc1-3 was subcultured in liquid LB medium for five consecutive rounds with or without antibiotic selection pressure. The restricted plasmids from 1st, 3rd, and 5th subcultures indicated that the copy number decreased during the continuous subculture if no zeocin was added, but the normal concentration of zeocin (25 mg/L) was sufficient to maintain a relatively stable copy number (Fig. 2D). But we could still see a little bit collapse of high-copy tandem duplication after five subculture (33 generations), so based on electrophoresis image, “intensity of high-copy-tandem-duplication band (top)/intensity of plasmid backbone band (bottom)” was used to determine relative stability of high-copy-tandem-duplication (relative to starting culture (0)) (Figure S1). This analysis showed that subculture with 33 generations still had 90% of high-copy-tandem-duplication band intensity of starting culture under normal concentration of antibiotics. To our knowledge, TDAH is the first in vivo technique for high-copy tandem duplication of plasmid DNA stabilized by normal concentration of antibiotics. Classical homology-mediated methods require a very high inducing concentration to stabilize the copy number, which is obviously not suitable for routine bacterial cultivation, but stabilization of copy number without selective agent could be achieved by subsequent knockout of endogenous recA (Tyo et al. 2009).
However, the copy number of Hc1 strain is bound to decline after long-term subcultivation without antibiotic selection pressure. So we extended the Hc1 to 70 generations without antibiotic selection pressure, and we found that the copy number of 70th subculture [CN(70)] was 2 and copy number of Hc1 starting culture [CN(0)] was 10 [CN(0)-CN(70)]/70 = 0.11, which means average 0.11 copies lost per cell per generation during long-term subcultivation (Table 2).
To apply TDAH to regulate gene expression, GFP was inserted as the GOI into the pOr1 plasmid. The fluorescence intensity analysis indicated that the GOI expression was enhanced as the tandem-duplication copy number increased in TDAH strains (Fig. 2E).
TDAH for E. coli genome–integrated DNA
Transgenes that are integrated into the host genome are generally more stable. We therefore developed a TDAH protocol for E. coli genome–integrated DNA (Fig. 3). Construction of the repeating unit was very similar to pOr1, but to obtain sufficient expression and dosage effect of the genome-integrated selectable marker gene, ble expression was driven by the Prrn promoter (Li and Bock 2019). The repeating unit was inserted into the lac operon locus of the genome to form the starting strain for TDAH, which was named Or2 (Fig. 3A). The procedures for the selection and identification of the tandem-duplication evolved strain (Ev2) and high-copy strain (Hc2) were the same as above. Quantitative analysis showed that the copy number of Ev2 was 2, as expected, while the Hc2 strains had from 6 to 17 copies. Southern blot analysis showed that the tandem-duplication regions in the Hc2 strains contained only the repeating units with different copy numbers (Fig. 3B, C). Then, the highest-copy-number strain Hc2-6 was subcultured for five consecutive rounds (approximate 33 generations) without antibiotic selection pressure, and we did not find any significant changes in the copy number (Fig. 3D). Thus, the genome-integrated high-copy DNA obtained by TDAH could remain relatively stable during continuous subculture even without antibiotic selection pressure or recA deletion. However, the copy number of Hc strain is bound to decline after long-term subcultivation without antibiotic selection pressure. By the same way as collapse rate analysis for Hc1, collapse rate of copy number of Hc2 was 0.04, which means average 0.04 copies lost per cell per generation during long-term subcultivation (Table 2). Average number of copies lost per cell per generation indicated that tandem duplication collapsed much slower than the plasmid per generation during long-term subcultivation. The copy number of Hc1 collapsed about threefold faster than that of Hc2. Quantitative analysis of the GFP mRNA levels in Hc2-6 also indicated that the high-copy strain had higher expression of the GOI (Fig. 3D). The mutant frequency of Ev2 was also calculated and was found to be approximately 6.3 × 10−11, which meant approximately 2.7 Ev2 colonies could be selected from 100 mL of Or2 culture (Table 1).
TDAH also works in Bacillus subtilis
Since the results showed that TDAH worked well in Gram-negative E. coli, we also tested its effect in the Gram-positive model bacterium Bacillus subtilis. The pOr3 vector was constructed for TDAH of plasmid DNA in Bacillus subtilis. The procedures or the selection and identification of the tandem-duplication evolved strain (Ev3) and high-copy strain (Hc3) were the same as in E. coli. The complete ble cassette (Cb) could be identified in Ev3 (Fig. 4B), and higher copy numbers were selected in Hc3 strains, from 3 to 8 copies (Fig. 4C). Thus, it was demonstrated that TDAH also works in Bacillus subtilis. So this indicates that the promoter capture strategy and this TDAH technique for DNA tandem duplication could work well in different bacterium species. We also calculated the mutant frequency of Ev3, which was found to be approximately 1.3 × 10−7, which meant that approximately 2419 Ev3 colonies could be selected from 100 mL of Or3 culture (Table 1).
Selected multi-copy DNA tandem duplication of plasmid DNA in Bacillus subtilis using the TDAH technique. A Schematic map of the repeating unit in the TDAH vector pOr3 for tandem duplication of Bacillus subtilis plasmid sequences. The length of the repeating unit was 1.6 kb. Primers used for PCR and DNA sequencing are indicated by arrows. The ble gene promoter (Pb3) and its matching terminator (Tb3) were derived from the expression system sp110. B Identification of the tandem-duplication evolutionary strain (Ev3). Ev3 could be selected by zeocin and identified by PCR with ble-fp and ble-rp (one 0.5-kb fragment). C Identification of the high-copy strain (Hc3). Hc3 could be selected by higher concentrations of zeocin. Tandem-duplication copy numbers of ble were measured by qPCR
recA deletion reduced the precision and efficiency of tandem duplication
As mentioned in the introduction, different from the existing in vivo tandem duplication methods, the TDAH construction does not contain any reported functional elements of homologous recombination and DNA amplification. recA is an essential component required for the previously reported homologous-recombination tandem duplication methods. To investigate the role of recA on tandem duplication in TDAH, we chose the recA-deficient E. coli strain DH5α as the host cell for TDAH. The pOr1 plasmid (TDAH for the wild-type E. coli strain MG1655) was introduced into DH5α to construct the starting strain for recA-free, which was called Or4. And Or1 and Or4 had similar efficiency of target recombination (formation of the correct ble cassette) (Fig. 5A, Table 1). Next, we conducted the restriction analysis to test Ev4 (Fig. 5B), and the various band patterns indicated that recombination of Ev4 took place in a chaotic manner. The above results suggested that the knockout of recA reduces the precision of tandem duplication. Given that except recA DH5α also has several other mutations, which possibly affected duplication, we knocked out the whole open reading frame of recA gene from E. coli MG1655 and inserted the repeating unit into the lac operon locus (same as Or2) to form Or5. In order to eliminate influence of plasmid copy number in tandem duplication efficiency, we also studied the impact of recA on genome-integrated DNA tandem duplication efficiency. The tandem-duplication evolved strain from Or5 could also be generated using the same protocol as described for Or2, but the frequency of Ev5 significantly decreased compared to Ev2 (Table 1). DNA sequencing result indicated the recombination of Ev5 was precise (Figs. 5C). The restriction analysis also supported that the fragment arrangement of Ev5 was precise (Fig. 5D). The results above showed that recA was non-essential to target recombination (formation of the correct ble cassette) but affected the efficiency and precision of tandem duplication.
recA deletion reduced the precision and efficiency of tandem duplication. A Identification of the tandem-duplication evolved strain (Ev4) from recA-deficient starting strain (DH5α). The pOr1 plasmid was introduced into the recA-deficient E. coli strain DH5α, resulting in the original strain Or4. Ev4 could also be selected using the same protocol as Ev1. B Identification of recombinational precision for Ev4. The plasmids of Ev4 were digested with SmaI, which cut once in the repeating unit. The various band patterns indicate that recombinations of Ev4 take place in a chaotic manner. C Identification of the Ev5 from recA-deletion MG1655 as starting strain. DNA sequencing result indicated that the recombination of Ev5 was precise. D Identification of recombinational precision for Ev5. The genomic DNA of Ev5 was digested with SmaI and HindIII, and the Southern blot analysis was hybridized with a ble probe. Red rectangle indicates the expected 1.6-kb fragment of repeat unit
Application of TDAH in selecting the optimal high copy number of ribA for vitamin B2 production in E. coli
We also applied TDAH to select the optimal high copy number of ribA for vitamin B2 production in E. coli (Fig. 6A). High copy number of ribA (Hc6) was selected from single-copy starting strain (Or6). The copy number of Hc6 selected from 200 mg/L zeocin could reach up to 6 (Figure S2A), but after several rounds of subculture, the copy number would decline rapidly until stabilizing at 3 even still with selection of 200 mg/L zeocin (Fig. 6B), Under the same circumstance of cultivation, Hc6 initially selected is more yellow than its subculture, indicating more vitamin B2 synthesized in higher-copy Hc6 strain than in 3-copy Hc6 strain (Figure S2B). However, E. coli with high-copy ribA is not stable in current culture condition, which may be due to adverse effect of excessive ribA on E. coli growth. Three-copy Hc6 strain was relatively stable and could generate vitamin B2 3.5-fold more than single-copy starting strain (Fig. 6C, D).
Application of TDAH in selecting the optimal high copy number of ribA for vitamin B2 production in E. coli. A Schematic map of TDAH vector pOr6. GFP gene of pOr1 was replaced by ribA gene driven by an artificial moderately strong promoter (PWGAN-1–4), producing pOr6. Total length of pOr6 is 4 kb. The length of repeating unit is 1.4 kb, and the restriction sites (KpnI and AscI) are located outside of the repeating unit. B Restriction analysis of Hc6. After several rounds of subculturing with selection of 200 mg/L zeocin, plasmid of Hc6 was restricted by KpnI and AscI, indicating 3 was a relatively stable copy number of ribA under this culture condition. C Vitamin B2 production of Or6 (single copy) and Hc6 (3 copies). D Restriction analysis for stability of copy number of Hc6. Hc6 was subcultured for 12 rounds with normal zeocin pressure (25 mg/L). The plasmids from Hc6 and its 12th subculture were restricted by KpnI and AscI
Discussion
In this study, we developed a simple, fast, and stable technique for tandem gene duplication based on our previous finding that spontaneous tandem DNA duplication resulted in rapid HTG activation. The TDAH construction only contains an essential selectable marker gene split at the flanks of the gene of interest, resulting in a very streamlined protocol, and the copy number could be relatively stable needless of subsequent recA knockout.
Tandem gene duplication is a focus of research on genome evolution and is widely used in gene expression regulation and biosynthetic pathway optimization. Thus, a powerful technique for tandem gene duplication is very important for basic and synthetic biology. Compared with the existing in vivo tandem duplication methods, high-copy tandem-duplicated plasmid DNA obtained by TDAH could be stabilized by normal concentration of antibiotics. Classical homology-mediated or Cre/loxP-mediated methods need a very high inducing concentration to stabilize the copy number, which is obviously not suitable for routine bacterial cultivation. To our knowledge, TDAH is the first in vivo technique for high-copy tandem duplication of plasmid DNA stabilized by normal concentrations of antibiotics. The copy number of the plasmid per cell could be very high, so the stable multi-copy tandem duplication for plasmid DNA will make the copy number of plasmid-carried GOIs extremely high. In this study, we used high-copy pUC57 as the backbone for pOr1, implying that its copy number per E. coli cell should be approximately 600. As there were more than 10 tandem-duplicated copies of the GOI per plasmid in Hc1 (Fig. 2C), the total number of expression cassettes can reach approximately 6000 per cell. In addition, TDAH was also successfully used to obtain the table high-copy tandem duplication of genome-integrated DNA in E. coli. The highest copy number from one round of high-concentration-antibiotic selection was approximately 17, and the corresponding total length of the tandem-duplication region was approximately 27 kb (1.6 × 17). We believe that this is still not the upper limit of the TDAH protocol. A higher copy number should be able to be obtained by more rounds of selection or higher inducing concentrations. The genome-integrated high-copy DNA obtained by TDAH remained stable even without antibiotic pressure. However, recA affected the efficiency and accuracy of tandem duplication. By contrast, the existing in vivo tandem duplication methods usually need the deletion of the endogenous recombination system to stabilize the high copy number. To our knowledge, TDAH should be the first reported stable and selected system which is needless of subsequent deletion of recombination system.
The reason why the high-copy tandem duplications are more stable than those obtained by other in vivo methods may be that TDAH uses a different and obscure molecular mechanism of tandem DNA duplication, which is supported by the fact that there are no reported DNA recombination and amplification elements in the TDAH construction. Thus, non-homologous mediates the tandem-duplication in the TDAH system. Researchers have found that many naturally occurring bacterial DNA duplication events were not mediated by sequence identity (Reams and Neidle 2004; Brochet et al. 2008; Reams et al. 2010; Lin et al. 2011; Sun et al. 2012; Elliott et al. 2013), indicating the importance of illegitimate recombination in bacterial DNA duplication. However, the evolutionary benefits and mechanisms of these illegitimate recombination events are still incompletely understood. Our previous study (Li and Bock 2019) and this work indicate that one of the evolutionary benefits would be the activation of previously silent horizontally transferred genes and the optimization of their expression. Nevertheless, bacterial illegitimate recombination is highly complex and still somewhat obscure. Researchers have found that many Rec proteins (RecE, RecF, RecJ, RecO, RecR, RecQ, etc.) participate in the regulation of illegitimate recombination in bacteria (Ukita and Ikeda 1996; Hanada et al. 1997; Shimizu et al. 1997; Shiraishi et al. 2002), but it is still not clear which could regulate the illegitimate recombination in TDAH or during the rapid activation of horizontally transferred genes. To our knowledge, TDAH is the first DNA duplication technique based on illegitimate recombination, and although illegitimate recombination was considered to be inefficient, uncontrollable, and unstable, TDAH offers high efficiency and stability. Because the TDAH construction does not contain any functional elements used in other in vivo methods and is based on a different mechanism, joint use of TDAH and other in vivo methods may further improve the efficiency of tandem duplication. This would require the addition of another selectable marker gene (split) flanking the original repeating unit, which will not affect the original tandem duplication system and is easy to construct. However, the combination of TDAH with other methods is beyond the scope of this paper and should be investigated in future studies.
The promoter capture strategy and this TDAH technique for DNA tandem duplication TDAH should be applicable in most laboratory strains and industrial hosts. We also found evidence that the molecular mechanism underlying TDAH should be conserved among bacteria, as it worked well both in Gram-negative E. coli and Gram-positive Bacillus subtilis. This indicates that the application of TDAH cloud also be extended to other organisms, such as yeasts, fungi, and even mammalian cell lines. This is easy to test, because TDAH is simple and fast, and the only technical requirement is a genetic transformation method and selection system. Interestingly, we found the efficiency of tandem duplication evolution using TDAH in B. subtilis was four orders of magnitude higher than that of E. coli. We speculated that this may be due to the differences in the mutation rate or the regulation details of illegitimate recombination between these two species. One important application of the tandem DNA duplication technique is the regulation of gene expression, which is widely used in recombinant protein production and expression optimization of biosynthetic pathways (Tyo et al. 2009; Yin et al. 2015; Peng et al. 2022). In this study, TDAH also showed a powerful ability to regulate gene expression. As expected, GOI expression was enhanced as the tandem-duplication copy number increased in TDAH strains (Figs. 2E and 3D). The scope of copy number variation was rather wide (Figs. 2C and 3C) and can be further tuned by subcultivation of plasmid-carried high-copy tandem-duplicated strains without the selection pressure (Fig. 2D). Thus, TDAH can potentially also be used to develop a library of strains with varying expression levels for one gene or multi-gene combinations. TDAH is therefore expected to become a powerful tool for in vivo tandem duplication of plasmid-carried and genome-integrated genes that is selected, stable, widely applicable, simple, and fast.
During the selection of the optimal high copy number of ribA for vitamin B2 production. ribA copy number initially reached to 6 and then rapidly declined until stabilizing at 3. Although a positive correlation was observed between ribA copy number and vitamin B2 biosynthesis, E. coli with high-copy ribA is not stable in current culture condition, which may be due to adverse effect of excessive ribA on E. coli growth. As reported, NADH is essential for vitamin B2 biosynthesis (Abbas and Sibirny 2011, Liu et al. 2021), but NADH generation pathway was not improved corresponsively in Hc6. The imbalance between the large biosynthesis flux of vitamin B2 and the intracellular limited NADH adversely affected cell growth and resulted in a decrease in ribA copy number. Initial high copy number could be fixed by recA deletion, but this may not lead to ultimate vitamin B2 production of E. coli culture improved and stable; we speculate that vitamin B2 biosynthesis driven by 3-copy ribA is maximal biosynthesis flux, which intracellular NADH could support in current culture condition; thereby, 3 is the optimal copy number of ribA for vitamin B2 production in our experiment.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Abbas CA, Sibirny AA (2011) Genetic control of biosynthesis and transport of riboflavin and flavin nucleotides and construction of robust biotechnological producers. Microbiol Mol Biol Rev 75(2):321–360. https://doi.org/10.1128/mmbr.00030-10
Anderson RP, Roth JR (1978) Tandem chromosomal duplications in Salmonella typhimurium: fusion of histidine genes to novel promoters. J Mol Biol 119:147–166
Averianova LA, Balabanova LA, Son OM, Podvolotskaya AB, Tekutyeva LA (2020) Production of vitamin B2 (riboflavin) by microorganisms: an overview. Front Bioeng Biotechnol 8:570828–570851. https://doi.org/10.3389/fbioe.2020.570828
Bacher A, Eberhardt S, Fischer M, Kis K, Richter G (2000) Biosynthesis of vitamin B2 (riboflavin). Annu Rev Nutr 20:153–167. https://doi.org/10.1146/annurev.nutr.20.1.153
Blount ZD, Barrick JE, Davidson CJ, Lenski RE (2012) Genomic analysis of a key innovation in an experimental Escherichia coli population. Nature 489:513–518. https://doi.org/10.1038/nature11514
Bock R (2017) Witnessing genome evolution: experimental reconstruction of endosymbiotic and horizontal gene transfer. Annu Rev Genet 51:1–22. https://doi.org/10.1146/annurev-genet-120215-035329
Boucher Y, Douady CJ, Papke RT, Walsh DA, Boudreau MER, Nesbo CL, Doolittle WF (2003) Lateral gene transfer and the origins of prokaryotic groups. Annu Rev Genet 37:283–328. https://doi.org/10.1146/annurev.genet.37.050503.084247
Brochet M, Couve E, Zouine M, Poyart C, Glaser P (2008) A naturally occurring gene amplification leading to sulfonamide and trimethoprim resistance in Streptococcus agalactiae. J Bacteriol 190(2):672–680. https://doi.org/10.1128/jb.01357-07
Brockhurst MA, Colegrave N, Rozen DE (2011) Next-generation sequencing as a tool to study microbial evolution. Mol Ecol 20(5):972–980. https://doi.org/10.1111/j.1365294X.2010.04835.x
Chen R, Guo H, Shen Y, Hu Y, Su Y (2006) Determination of EOF of PMMA microfluidic chip by indirect laser-induced fluorescence detection. Sens Actuators B Chem 114(2):1100–1107. https://doi.org/10.1016/j.snb.2005.07.047
Dagan T, Artzy-Randrup Y, Martin W (2008) Modular networks and cumulative impact of lateral transfer in prokaryote genome evolution. PNAS 105(29):10039–10044. https://doi.org/10.1073/pnas.0800679105
Elliott KT, Cuff LE, Neidle EL (2013) Copy number change: evolving views on gene amplification. Future Microbiol 8(7):887–899. https://doi.org/10.2217/fmb.13.53
Ginty SEM, Rankin DJ, Brown SP (2011) Horizontal gene transfer and the evolution of bacterial cooperation. Evolution 65(1):21–32. https://doi.org/10.1111/j.1558-5646.2010.01121.x
Gogarten JP, Doolittle WF, Lawrence JG (2002) Prokaryotic evolution in light of gene transfer. Mol Biol Evol 19(12):2226–2238. https://doi.org/10.1093/oxfordjournals.molbev.a004046
Hanada K, Ukita T, Kohno Y, Saito K, Kato J, Ikeda H (1997) RecQ DNA helicase is a suppressor of illegitimate recombination in Escherichia coli. PNAS 94(8):3860–3865. https://doi.org/10.1073/pnas.94.8.3860
Katju V, Bergthorsson U (2013) Copy-number changes in evolution: rates, fitness effects and adaptive significance. Front Genet 4:273. https://doi.org/10.3389/fgene.2013.00273
Lan RT, Reeves PR (1996) Gene transfer is a major factor in bacterial evolution. Mol Biol Evol 13(1):47–55. https://doi.org/10.1093/oxfordjournals.molbev.a025569
Li Z, Bock R (2019) Rapid functional activation of a horizontally transferred eukaryotic gene in a bacterial genome in the absence of selection. Nucleic Acids Res 47(12):6351–6359. https://doi.org/10.1093/nar/gkz370
Li C, Ng MLP, Zhu Y, Ho B, Ding JL (2003) Tandem repeats of Sushi3 peptide with enhanced LPS-binding and -neutralizing activities. Protein Eng Des Sel 16(8):629–635. https://doi.org/10.1093/protein/gzg078
Lin Z, Xu Z, Li Y, Wang Z, Chen T, Zhao X (2014) Metabolic engineering of Escherichia coli for the production of riboflavin. Microb Cell Fact 13:1–12. https://doi.org/10.1186/s12934-014-0104-5
Lin D, Gibson IB, Moore JM, Thornton PC, Leal SM, Hastings PJ (2011) Global Chromosomal structural instability in a subpopulation of starving Escherichia coli cells. Plos Genet 7(8). https://doi.org/10.1371/journal.pgen.1002223
Liu D, Mao Z, Guo J, WeiL MH, Tang Y, Zhao X (2018) Construction, model-based analysis, and characterization of a promoter library for fine-tuned gene expression in Bacillus subtilis. Acs Synth Biol 7(7):1785–1797. https://doi.org/10.1021/acssynbio.8b00115
Liu S, Hu W, Wang Z, Chen T (2021) Rational engineering of Escherichia coli for high-level production of riboflavin. J Agric Food Chem 69(41):12241–12249. https://doi.org/10.1021/acs.jafc.1c04471
Matus-Garcia M, Nijveen H, Van Passel MWJ (2012) Promoter propagation in prokaryotes. Nucleic Acids Res 40(20):10032–10040. https://doi.org/10.1093/nar/gks787
Ochman H, Lawrence JG, Groisman EA (2000) Lateral gene transfer and the nature of bacterial innovation. Nature 405(6784):299–304. https://doi.org/10.1038/35012500
Oren Y, Smith MB, Johns NI, Zeevi MK, Biran D, Ron EZ, Pupko T (2014) Transfer of noncoding DNA drives regulatory rewiring in bacteria. PNAS 1011(45):16112–16117. https://doi.org/10.1073/pnas.141327211
Pardo I, Bedore SR, Tumen-Velasquez MP, Duscent-Maitland CV, Baugh AC, Santala S, Neidle EL (2023) Natural transformation as a tool in Acinetobacter baylyi: Evolution by amplification of gene copy number. Method Microbiol 52:183–205. https://doi.org/10.1016/bs.mim.2023.01.001
Peng B, Esquirol L, Lu Z, Shen Q, Cheah LC, Howard CB, Vickers CE (2022) An in vivo gene amplification system for high level expression in Saccharomyces cerevisiae. Nat Commun 13(1):2895. https://doi.org/10.1038/s41467-022-30529-8
Popa O, Dagan T (2011) Trends and barriers to lateral gene transfer in prokaryotes. Curr Opin Microbiol 14(5):615–623. https://doi.org/10.1016/j.mib.2011.07.027
Popa O, Hazkani-Covo E, Landan G, Martin W, Dagan T (2011) Directed networks reveal genomic barriers and DNA repair bypasses to lateral gene transfer among prokaryotes. Genome Res 21(4):599–609. https://doi.org/10.1101/gr.115592.110
Rao X, Hu J, Li Sh, Jin X, Zhang Ch, Cong Y, Hu X, Tan Y, Huang J, Chen Z, Zhu J, Hu F (2005) Design and expression of peptide antibiotic hPAB-β as tandem multimers in Escherichia coli. Peptides 26(5):721–729. https://doi.org/10.1016/j.peptides.2004.12.016
Reams AB, Neidle EL (2004) Gene amplification involves site-specific short homology-independent illegitimate recombination in Acinetobacter sp. Strain ADP1. J Mol Biol 338(4):643–656. https://doi.org/10.1016/j.jmb.2004.03.031
Reams AB, Kofoid E, Savageau M, Roth JR (2010) Duplication frequency in a population of Salmonella enterica rapidly approaches steady state with or without recombination. Genetics 184(4):1077–1094. https://doi.org/10.1534/genetics.109.111963
Shimizu H, Yamaguchi H, Ashizawa Y, Kohno Y, Asami M, Kato J, Ikeda H (1997) Short-homology-independent illegitimate recombination in Escherichia coli: distinct mechanism from short-homology-dependent illegitimate recombination. J Mol Biol 266(2):297–305. https://doi.org/10.1006/jmbi.1996.0794
Shiraishi K, Hanada K, Iwakura Y, Ikeda H (2002) Roles of recJ, RecO, and RecR in RecET-mediated illegitimate recombination in Escherichia coli. J Bacteriol 184(17):4715–4721. https://doi.org/10.1128/jb.184.17.4715-4721.2002
Stegemann S, Bock R (2006) Experimental reconstruction of functional gene transfer from the tobacco plastid genome to the nucleus. Plant Cell 18(11):2869–2878. https://doi.org/10.1105/tpc.106.046466
Sun S, Ke R, Hughes D, Nilsson M, Andersson DI (2012) Genome-wide detection of spontaneous chromosomal rearrangements in bacteria. PLoS ONE 7(8):e42639. https://doi.org/10.1371/journal.pone.0042639
Thomas CM, Nielsen KM (2005) Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Microbiol 3(9):711–721. https://doi.org/10.1038/nrmicro1234
Tian Z, Teng D, Yang Y, Luo J, Wang J (2007) Multimerization and fusion expression of bovine lactoferricin derivative LFcinB15-W4,10 in Escherichia coli. Appl Microbiol Biot 75(1):117–124. https://doi.org/10.1007/s00253-006-0806-7
Treangen TJ, Rocha EP (2011) Horizontal transfer, not duplication, drives the expansion of protein families in prokaryotes. Plos Genet 7(1):e1001284. https://doi.org/10.1371/journal.pgen.1001284
Tyo KEJ, Ajikumar PK, Stephanopoulos G (2009) Stabilized gene duplication enables long-term selection-free heterologous pathway expression. Nat Biotechnol 27(8):760–765. https://doi.org/10.1038/nbt.1555
Ukita T, Ikeda H (1996) Role of the recJ gene product in UV-induced illegitimate recombination at the hotspot. J Bacteriol 178(8):2362–2367. https://doi.org/10.1128/jb.178.8.2362-2367.1996
Wang Y, Wang H, Wei L, Li S, Liu L, Wang X (2020) Synthetic promoter design in Escherichia coli based on a deep generative network. Nucleic Acids Res 48(12):6403–6412. https://doi.org/10.1093/nar/gkaa325
Wu C, Valdes JJ, Rao G, Bentley WE (2010) Enhancement of organophosphorus hydrolase yield in Escherichia coli using multiple gene fusions. Biotechnol Bioeng 75(1):100–103. https://doi.org/10.1002/bit.1169
Yin J, Wang H, Fu X, Gao X, Wu Q, Chen G (2015) Effects of chromosomal gene copy number and locations on polyhydroxyalkanoate synthesis by Escherichia coli and Halomonas sp. Appl Microbiol Biot 99(13):5523–5534. https://doi.org/10.1007/s00253-015-6510-8
Zhao D, Feng X, Zhu X, Wu T, Zhang X, Bi C (2017) CRISPR/Cas9-assisted gRNA-free one-step genome editing with no sequence limitations and improved targeting efficiency. Sci Rep 7:1–9. https://doi.org/10.1038/s41598-017-16998-8
Acknowledgements
We thank Professor Changhao Bi (Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences) for providing lambda Red recombination system and Dr. Dingyu Liu (Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences) for providing expression system of Bacillus subtilis.
Funding
This work was supported by the National Key R&D Program of China (2020YFA0908000), Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-CXRC-001), and key project at central government level: The ability establishment of sustainable use for valuable Chinese medicine resources (2060302).
Author information
Authors and Affiliations
Contributions
F.Z. performed most of the experiments and analyzed data. X.S. performed all real-time PCR experiments, analyzed data, and wrote the manuscript. J.X. performed parts of the experiments and wrote the manuscript. W.Y. carried out part of vector construction work. Z.L. conceived of the project, analyzed data, and wrote the manuscript.
Corresponding author
Ethics declarations
Declarations
The authors declare no competing interests and this article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, F., Shi, X., Xu, J. et al. Tandem gene duplication selected by activation of horizontally transferred gene in bacteria. Appl Microbiol Biotechnol 108, 340 (2024). https://doi.org/10.1007/s00253-024-13160-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13160-z