Summary
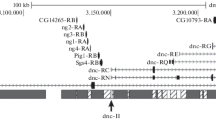
We have used 160 kilobases of cloned Drosophila genomic DNA from the rudimentary (r) region to examine the organization of amplified DNA in Drosophila cells resistant to 10 mM N-(phosphonacetyl)-l-aspartate (PALAr cells) obtained by stepwise selection. Evidence for the direct tandem linkage of the amplified sequences is presented. The pattern and intensity of amplified bands as well as the presence of novel junctions in the DNA sequence of PALAr cells indicate that there are two types of units of 150 and 120 kilobases long. The sequence of the smaller unit is entirely included within the larger one. The longer of the two units is present twice while the shorter one is amplified eightfold as compared to the level of the relevant DNA sequences in the wild-type. These data are consistent with a model in which successive crossing-over events over several cell cycles lead to amplification of the selected r gene and flanking sequences. However, these data can also be accounted for by a totally different mechanism in which multiple copies of DNA are generated by rolling circle replication. Transcription units other than the r gene are present within the 150 kilobase region of amplified DNA. These are found to be overexpressed in PALA' cells, though some transcripts are underrepresented relative to the copy number of the corresponding coding sequences.
Similar content being viewed by others
References
Anderson RP, Roth JR (1977) Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol 31:473–504
Anderson RP, Roth JR (1981) Spontaneous tandem genetic duplications in Salmonella typhimurium arise by unequal recombination between rRNA (rrn) cistrons. Proc Natl Acad Sci USA 78:3113–3117
Auffray C, Rougeon F (1980) Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem 107:303–314
Bantle JA, Maxwell IH, Hahn WE (1976) Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem 72:413–427
Benton WD, Davis RW (1977) Hybridization in situ of SV40 plaques: detection of recombinant SV40 virus carrying specific sequences of nonviral DNA. Science 196:180–182
Blackwell TK, Moore MW, Yancopoulos GD, Suh H, Lutzker S, Selsing E, Alt FW (1986) Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature 324:585–589
Debatisse M, Robert de Saint Vincent B, Buttin G (1984) Expression of several amplified genes in an adenylate-deaminase overproducing variant of Chinese hamster fibroblasts. EMBO J 3:3123–3127
Debatisse M, Hyrien O, Petit-Koskas E, Robert de Saint-Vincent B, Buttin G (1986) Segregation and rearrangement of coamplified genes in different lineages of mutant cells that overproduce adenylate deaminase. Mol Cell Biol 6:1776–1781
de Bruijn MHL, van der Blick AM, Biedler JL, Borst P (1986) Differential amplification and disproportionate expression of five genes in three multidrug-resistant Chinese hamster lung cell lines. Mol Cell Biol 6:4717–4722
de Cicco DV, Glover DM (1983) Amplification of rDNA and type I sequences in Drosophila males deficient in rDNA. Cell 32:1217–1225
Edlund T, Normark S (1981) Recombination between short DNA homologies causes tandem duplication. Nature 292:269–271
Endow SA, Atwood KC (1988) Magnification: gene amplification by an inducible system of sister chromatid exchange. Trends Genet 4:348–351
Ford M, Fried M (1986) Large inverted duplications are associated with gene amplification. Cell 45:425–430
Freund JN, Vergis W, Schedl P, Jarry BP (1986) Molecular organization of the rudimentary gene of Drosophila melanogaster. J Mol Biol 189:25–36
Fyrberg EA, Kindle KL, Davidson N, Sodja A (1980) The actin genes of Drosophila: a dispersed multigene family. Cell 19:365–378
Gilbert W, Dressler D (1968) DNA replication: the rolling circle model. Cold Spring Harbor Symp Quant Biol 33:473–477
Giorgi D, Laval M, Pardo D (1983) Amplification of the rudimentary gene in a PALA-resistant Drosophila cell line. FEBS Lett 162:374–378
Giulotto E, Saito I, Stark G (1986) Structure of DNA formed in the first step of CAD gene amplification. EMBO J 5:2115–2121
Goldberg DA (1980) Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci USA 77:5794–5798
Hamlin JL, Milbrandt JD, Heintz NH, Azizkhan JC (1984) DNA sequence amplification in mammalian cells. Int Rev Cytol 90:31–82
Hyrien O, Debatisse M, Buttin G, Robert de Saint Vincent B (1988) The multicopy appearance of a large inverted duplication and the sequence at the inversion joint suggest a new model for gene amplification. EMBO J 7:407–417
Kafatos FC, Orr W, Delikadis C (1985) Developmentally regulated gene amplification. Trends Genet 1:301–306
Kaufman RJ, Brown PC, Schimke RT (1979) Amplified dihydrofolate reductase genes in unstably methotrexate-resistant cells are associated with double minute chromosomes. Proc Natl Acad Sci USA 76:5669–5673
Klar AJS, Strathern JN, Abraham JA (1984) Involvement of double-strand chromosomal breaks for mating-type switching in Saccharomyces cerevisiae. Cold Spring Harbor Symp Quant Biol 49:77–88
Laval M, Azou Y, Giorgi D, Rosset R (1986) Overproduction of the first three enzymes of pyrimidine nucleotide biosynthesis in Drosophila cells resistant to N-phosphonacetyl-L-aspartate. Exp Cell Res 163:381–395
Looney JE, Hamlin JL (1987) Isolation of the amplified dihydrofolate reductase domain from methotrexate-resistant Chinese hamster ovary cells. Mol Cell Biol 7:569–577
Looney JE, Ma C, Len TH, Flintoff WF, Troutman WB, Hamlin JL (1988) The dihydrofolate reductase amplicons in different methotrexate-resistant Chinese hamster cell lines share at least a 273-kilobase core sequence, but the amplicons in some cell lines are much larger and are remarkably uniform in structure. Mol Cell Biol 8:5268–5279
Maniatis T, Hardison RC, Lacy E, Lauer J, O'Connell C, Quon D, Sim GK, Efstratiadis A (1978) The isolation of structural genes from libraries of eucaryotic DNA. Cell 15:687–701
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Nalbantoglu J, Meuth M (1986) DNA amplification-deletion in a spontaneous mutation of the hamster aprt locus: structure and sequence of the novel joint. Nucleic Acids Res 14:8361–8371
Otto E, Young JE, Maroni G (1986) Structure and expression of a tandem duplication of the Drosophila metallothionein gene. Proc Natl Acad Sci USA 83:6025–6029
Pall ML (1981) Gene amplification model of carcinogenesis. Proc Natl Acad Sci USA 78:2465–2468
Passananti C, Davies B, Ford M, Fried M (1987) Structure of an inverted duplication formed as a first step in a gene amplification event: implications for a model of gene amplification EMBO J 6:1697–1703
Rawls JM, Freund JN, Jarry BP, Louis C, Segraves WA, Schedl P (1986) Organization of transcription units around the Drosophila melanogaster rudimentary locus and temporal pattern of expression. Mol Gen Genet 202:493–499
Ritossa F, Malva C, Boncinelli E, Graziani F, Polito L (1971) The first steps of magnification of DNA complementary to ribosomal RNA in Drosophila melanogaster. Proc Natl Acad Sci USA 68:1580–1584
Rubin GM (1983) Dispersed repetitive DNAs in Drosophila. In: Shapiro JA (ed) Mobile genetic elements. Academic Press, New York, pp 329–361
Ruiz JC, Wahl GM (1988) Formation of an inverted duplication can be an initial step in gene amplification. Mol Cell Biol 8:4302–4313
Saito I, Stark GR (1986) Charomids: cosmid vectors for efficient cloning and mapping of large or small restriction fragments. Proc Natl Acad Sci USA 83:8664–8668
Schimke RT (1982) Summary. In: Schimke RT (ed) Gene amplification. Cold Spring Harbor Laboratory Press, New York, pp 317–333
Schimke RT (1984) Gene amplification in cultured animal cells. Cell 37:705–713
Segraves WA, Louis C, Schedl P, Jarry BP (1983) Isolation of the rudimentary locus of Drosophila melanogaster. Mol Gen Genet 189:34–40
Shiloh Y, Shipley J, Brodeur GM, Bruns G, Korf B, Donlon T, Schreck RR, Seeger R, Sakai K, Latt SA (1985) Differential amplification, assembly, and relocation of multiple DNA sequences in human neuroblastomas and neuroblastoma cell lines. Proc Natl Acad Sci USA 82:3761–3765
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Spradling AC, Mahowald AP (1980) Amplification of genes for chorion proteins during oogenesis in Drosophila melanogaster. Proc Natl Acad Sci USA 77:1096–1100
Stark GR, Wahl GM (1984) Gene amplification. Annu Rev Biochem 53:447–491
Stark GR, Debatisse M, Giulotto E, Wahl GM (1989) Recent progress in understanding mechanisms of mammalian DNA amplification. Cell 57:901–908
Tartof KD (1973) Regulation of ribosomal RNA gene multiplicity in Drosophila melanogaster. Genetics 73:57–71
Tartof KD (1974) Unequal mitotic sister chromatid exchange as the mechanism of ribosomal RNA gene magnification. Proc Natl Acad Sci USA 71:1272–1276
Terracol R, Prud'homme N (1986) Differential elimination of rDNA genes in bobbed mutants of Drosophila melanogaster. Mol Cell Biol 6:1023–1031
Thomas PS (1980) Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci USA 77:5201–5205
Tlsty TD, Albertini AM, Miller JH (1984) Gene amplification in the lac region of E. coli. Cell 37:217–224
van der Bliek AM, van der Velde-Koerts T, Ling V, Borst P (1986) Overexpression and amplification of five genes in a multidrugresistant Chinese hamster ovary cell line. Mol Cell Biol 6:1671–1678
Vaslet CA, O'Connel P, Izquierdo M, Rosbach M (1980) Isolation and mapping of a cloned ribosomal protein gene of Drosophila melanogaster. Nature 285:674–676
Voelkel-Meiman K, Keil RL, Roeder GS (1987) Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell 48:1071–1079
Wahl GM, Vitto L, Padgett RA, Stark GR (1982) Single-copy and amplified CAD genes in Syrian hamster chromosomes localized by a highly sensitive method for in situ hybridization. Mol Cell Biol 12:308–319
Wellauer PK, Dawid IB, Tartof KD (1978) X and Y chromosomal ribosomal DNA of Drosophila: comparison of spacers and insertions. Cell 14:269–278
White BA, Bancroft FC (1982) Cytoplasmic dot hybridization: simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem 257:8569–8572
Whoriskey SK, Nghiem VH, Leong PM, Masson JM, Miller JH (1987) Genetic rearrangement and gene amplification in Escherichia coli: DNA sequences at the junctures of amplified gene fusions. Genes Develop 1:227–237
Young M, Cullum J (1987) A plausible mechanism for large-scale chromosomal DNA amplification in streptomycetes. FEBS Lett 212:10–14
Author information
Authors and Affiliations
Additional information
Communicated by B.H. Judd
Rights and permissions
About this article
Cite this article
Laval, M., Azou, Y. & Miassod, R. Structural organization and expression of amplified chromosomal sequences, which include the rudimentary gene, in cultured Drosophila cells resistant to N-(phosphonacetyl)-l-aspartate. Molec. Gen. Genet. 220, 102–112 (1989). https://doi.org/10.1007/BF00260863
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00260863