Abstract
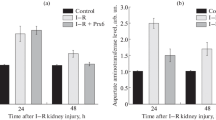
The results of our experiments demonstrated that one hour of ischemia followed by one hour of reflow in the kidney caused a reduction in (Na+K+)ATPase activity and microsomal sulfhydryl content as well as an increase in microsomal lipid peroxidation. Renal venous malondialdehyde concentration was increased soon after reperfusion of the ischemic kidney. All these changes were rectified by an infusion of 0.123 mmol N-(2-mercaptopropionyl)glycine/kg over a 70 min period. On the other hand, an in vitro addition of 0.01–0.5 mM N-(2-mercaptopropionyl)glycine to a membrane preparation in the presence of H202 and Fe3+ did not prevent but rather potentiated the free radical effect on the enzyme activity. However, addition of superoxide dismutase alone or with catalase together with 2-MPG were effective in preventing the enzyme depression induced by H202. The results therefore indicate that free radical generation participates in the evolution of ischemia/reperfusion cell injury and thiol-reducing agents may be beneficial in alleviating the cell damage in vivo.
Similar content being viewed by others
References
Chance B, Sies H, Boveris A: Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605, 1979
Freeman BA, Crapo JD: Biology of disease. Free radicals and tissue injury. Lab Invest 47:412–426, 1982
Weiss SJ, LoBuglio AF: Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest 47:5–18, 1982
McCord JM: Oxygen-derived free radicals in postischemic tissue injury. New Engl J Med 312:159–163, 1985
Werns SW, Shea MJ, Lucchesi BR: Free radicals in ischemic myocardial injury. J Free Rad Biol Med 1:103–110, 1985
Kako KJ: Membrane damage caused by lipid peroxidation in myocardial ischemia. Jikeikai Med J 32:609–639, 1985
Wolff SP, Garner A, Dean RT. Free radicals, lipids and protein degradation. TIBS 11:27–31, 1986
Kako KJ: Free radical effects on membrane protein in myocardial ischemia/reperfusion injury. J Mol Cell Cardiol 19:209–211, 1987
Venkatachalam MA, Bernard DB, Donohoe JF, Levinsky NG: Ischemic damage and repair in the rat proximal tubule. Differences among the Sl, S2 and S3 segments. Kidney Int 14:31–49, 1978
Brezis M, Rosen S, Silva P, Epstein FH: Renal ischemia. A new perspective. Kidney Int 26:375–383, 1984
Mason J, Thiel G (eds) Workshop on the role of renal medullary circulation in the pathogenesis of acute renal failure. Nephron 31:289–323, 1982
Kako KJ, Kato M, Matsuoka T, Mustapha A: The depression of membrane-bound (Na+K+)ATPase activity induced by free radicals and by ischemia of the kidney. Am J Physiol in press
Kako KJ, Kato M, Matsuoka T, Kaminishi T: Difference in in vitro effects of two oxidants, hydrogen peroxide and hypochlorite, on isolated membranes and cardiomyocytes. Abst J Mol Cell Cardiol 19, Suppl 4,S-78, 1987
Jorgensen PL: Sodium and potassium ion pump in kidney tubules. Physiol Rev 60:864–917, 1980
Williams R, Gasbarrini G, Davis M (eds). Recent advances in 2-MPG treatment of liver diseases. Edtrice Compositori Bologna 1–383, 1981
Mitsos SE, Askew TE, Fantone JC, Kunkel SL, Abrams GD, Schork A, Lucchesi BR: Protective effects of N-2-mercaptopropionyl glycine against myocardial reperfusion injury af neutrophil depletion in the dog: evidence for the role of intracellular-derived free radicals. Circulation 73:1077–1086, 1986
Myers M, Bolli R, Lekich RF, Hartley CJ, Roberts R: N-2-mercaptopropionylglycine improves recovery of myocardial function after reversible regional ischemia. J Am Coll Cardiol 8:1161–1168, 1986
Jorgensen PL: Purification and characterization of (Na+K+)ATPase. III. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate. Biochim Biophys Acta 356:36–52, 1974
Kako KJ, Zaror-Behrens G, Peckett SD: Phosphatidic acid synthesis in the heart. Effect of age and species difference in the mitochondrial and microsomal synthesis. Can J Biochem 55:308–314, 1977
Kayawake S, Narbaitz R, Kako KJ: Effects of chloroquine and Nifedipine on the phospholipid content and enzyme activity in the subcellular fraction of ischemic rat liver. Basic Res Cardiol 77:140–157, 1982
Takahashi K, Kako KJ: The effect of a calcium channel antagonist, Nisoldipine, on the ischemia-induced change of canine sarcolemmal membrane. Basic Res Cardiol 78:326–337, 1983
Peterson CL: A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356, 1977
Sakai H: Quantitative microdetermination of total -SH groups in proteins. Anal Biochem 26:269–276, 1968
Ohkawa H, Ohishi N, Yagi K: Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358, 1979
Weber K, Osborne M: The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem 244:4406–4412, 1969
Balint P, Szocs E: Intrarenal hemodynamics following temporary occlusion of the renal artery in the dog. Kidney Int 10, S-128–136, 1976
Karlberg L, Norlen BJ, Ojteg G, Wolgast M: Impaired medullary circulation in postischemic acute renal failure. Acta Physiol Scand 118:11–17, 1983
Zimmer G, Beyersdorf F, Fuchs J: Decay of structure and function of heart mitochondria during hypoxia and related stress and its treatment. Mol Physiol 8:495–513, 1985
Starke PE, Farber JL: Endogenous defenses against the cytotoxicity of hydrogen peroxide in cultured rat hepatocytes. J Biol Chem 260:86–92, 1985
Reeves JP, Bailey CA, Hale CC: Redox modification of sodium-calcium exchange activity in cardiac sarcolemmal vesicles. J Biol Chem 261:4948–4988, 1986
Paller MS, Hebbel RP: Ethane production as a measure of lipid peroxidation after renal ischemia. Am J Physiol 251:F839–843, 1986
Paller MS, Hoidal JR, Ferris TF: Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest 74:1156–1164, 1984
Garner A, Jamal Z, Slater TF: Effects of 2-mercaptopropionyl glycine on radiation-induced lipid peroxidation in liposomes and in rat liver microsomal suspensions. Int J Radiat Biol 50:323–335, 1986
Schraufstatter IU, Hyslop PA, Hinshaw DB, Spragg RG, Sklar LA, Cochrance CG: Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci 83:4908–4912, 1986
Altschuld RA, Wenger WC, Lamka KG, Kindig OR, Capen CC, Mizuhira V, Vander Heide RS, Brierley GP: Structural and functional properties of adult rat heart myocytes lysed with digitonin. J Biol Chem 260:14325–14334, 1985
Author information
Authors and Affiliations
Additional information
Dr. Kato was on leave from the Department of Medicine (Prof. M. Nagano), Jikei University-Aoto Hospital, Tokyo, and was supported by the Uehara Foundation.
Rights and permissions
About this article
Cite this article
Kato, M., Kako, K.J. Effects of N-(2-mercaptopropionyl)glycine on ischemic-reperfused dog kidney in vivo and membrane preparation in vitro . Mol Cell Biochem 78, 151–159 (1987). https://doi.org/10.1007/BF00229689
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00229689