Summary
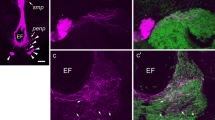
The heads of small adult snails, Helix aspersa, were injected with horseradish peroxidase (HRP) for one to five hours before extirpating the eyes and preparing them cytochemically for electron microscopy. There was internalization of tracer by pinocytic vesicles (pinosomes) at the bases of types-I and -II sensory cells, ganglion cells and, in lesser amounts, by pigmented supportive cells. Vesicles and vacuoles filled with HRP were transported in two directions: lensward as far distad as the ends of the cells (retrograde) and brainward down the optic nerve (anterograde). We believe that the numerous reacted vacuoles in the cell somata are formed by fusion of vesicles, tubules and C-shaped organelles filled with tracer; we present evidence that they become secondary lysosomes. Sensory cell type II possesses more HRP-reacted vacuoles distally than the other retinal cells. Other vesicles are also described. There was no uptake of tracer by the distal ends of the retinal cells following injection of HRP into the hemolymph. The swelling of the optic nerve, immediately behind the eye, contains more HRP-filled pinosomes and vacuoles than does the nerve below the dilatation. The significance of endocytosis and transport of pinosomes within the eye and down the optic nerve is discussed.
Similar content being viewed by others
References
Abrahamson DR, Rodewald R (1981) Evidence for the sorting of endocytic vesicle contents during the receptor-mediated transport of IgG across the newborn rat intestine. J Cell Biol 91:270–280
Blest AD, Price DG (1981) A new mechanism for transitory, local endocytosis in photoreceptors of a spider, Dinopis. Cell Tissue Res 217:267–282
Brandenburger JL (1975) Two new kinds of retinal cells in the eye of a snail, Helix aspersa. J Ultrastruct Res 50:216–230
Brandenburger JL (1977) Cytochemical localization of acid phosphatase in regenerated and dark-adapted eyes of a snail, Helix aspersa. Cell Tissue Res 184:301–313
Bunt AH, Lund RD, Lund JS (1974) Retrograde axonal transport of horseradish peroxidase by ganglion cells of the albino rat retina. Brain Res 73:215–228
Bunt AH, Haschke RH, Lund RD, Calkins DF (1976) Factors affecting retrograde axonal transport of horseradish peroxidase in the visual system. Brain Res 102:152–155
Eakin RM, Brandenburger JL (1970) Degeneration in severed optic nerve of a pulmonate snail, Helix aspersa. J Cell Biol 47:54a
Eakin RM, Brandenburger JL (1982) Pinocytosis in eyes of a snail, Helix aspersa. J Ultrastruct Res 80:214–229
Eakin RM, Brandenburger JL, Westree B (1970) Does the optic nerve of a snail, Helix aspersa, have a ganglion? Proc 7th Int Congr Electron Microsc (Grenoble) pp 673–674
Hansson HA (1973) Uptake and intracellular bidirectional transport of horseradish peroxidase tracers in retinal ganglion cells. Exp Eye Res 16:377–388
Holtzman E, Schacher S, Evans J, Teichberg S (1977) Origin and fate of the membranes of secretion granules and synaptic vesicles: membrane circulation in neurons, gland cells and retinal photoreceptors. In: Poste G, Nicolson GL (1977) Cell surface reviews. Vol 4: The synthesis, assembly, and turnover of cell surface components. Elsevier North Holland, Amsterdam, pp 165–246
Kirkpatrick JB, Bray JJ, Palmer SM (1972) Visualization of axoplasmic flow in vitro by Nomarski microscopy. Comparison to rapid flow of radioactive proteins. Brain Res 43:1–10
Krishnan N, Singer M (1973) Penetration of peroxidase into peripheral nerve fibers. Am J Anat 136:1–14
Kristensson K (1975) Retrograde axonal transport of protein tracers. In: Cowan WM, Cuénod M (eds) The use of axonal transport for studies of neuronal connectivity. Elsevier, Amsterdam, pp 71–82
Kristensson K, Olsson Y (1971) Uptake and retrograde axonal transport of peroxidase in hypoglossal neurones. Acta Neuropathol 19:1–9
Lane NJ, Swales LS (1976) Interrelationships between Golgi, GERL, and synaptic vesicles in the nerve cells of insect and gastropod ganglia. J Cell Sci 22:435–453
LaVail JH (1975) Retrograde cell degeneration and retrograde transport techniques. In: Cowan WM, Cuénod M (eds) The use of axonal transport for studies of neuronal connectivity. Elsevier, Amsterdam, pp 217–248
LaVail JH (1978) A review of the retrograde transport technique. In: Robertson RT (ed) Methods in physiological psychology. Vol 2: Neuroanatomical research techniques. Academic Press, New York, pp 356–384
LaVail JH, LaVail MM (1974) The retrograde intraaxonal transport of horseradish peroxidase in the chick visual system: A light and electron microscopic study. J Comp Neurol 157:303–358
Nässel DR (1982) Transneuronal uptake of HRP in the central nervous system of dipterous insects. Cell Tissue Res 225:639–662
Nauta HJW, Pritz MB, Lasek RJ (1974) Afferents to the rat caudoputamen studied with horseradish peroxidase. An evaluation of a retrograde neuroanatomical research method. Brain Res 67:219–238
Nichols BA (1981) Use of ultrastructural histochemistry. In: Adams DO, Edelson PJ, Koren (eds) Methods for studying mononuclear phagocytes. Academic Press, New York, pp 413–432
Prosser CL, Brown FA (1961) Comparative animal physiology. Saunders, Philadelphia, pp 1–688
Schacher S, Holtzman E, Hood DC (1976) Synaptic activity of frog retinal photoreceptors: A peroxidase uptake study. J Cell Biol 70:178–192
Sellinger OZ, Petiet PD (1973) Horseradish peroxidase uptake in vivo by neuronal and glial lysosomes. Exp Neurol 38:370–385
Teichberg S, Holtzman E, Crain SM, Peterson ER (1975) Circulation and turnover of synaptic vesicle membrane in cultured spinal cord neurons. J Cell Biol 67:215–230
Weiss DG (ed) (1982). Axoplasmic transport. Springer, Berlin Heidelberg New York, pp 1–477
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brandenburger, J.L., Eakin, R.M. Transport of pinocytic vesicles in the eye of a snail, Helix aspersa . Cell Tissue Res. 232, 35–52 (1983). https://doi.org/10.1007/BF00222372
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00222372