Summary
-
1.
Retinular fine structure in compound eyes of the porcellanid crab Petrolisthes differs significantly from two paguroid anomurans Clibanarius and Pagurus which basically conform to the usual conservative decapod crustacean retinular pattern.
-
2.
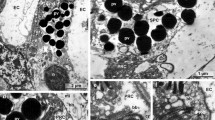
Bidirectional orientation of microvilli has been discovered in rhabdomeres of retinular cells R1–R7 in Petrolisthes. Distally the regular rhabdom has mainly a typical banded microvillus structure (Figs. 7, 8). Proximally rhabdom banding continues but uniquely all seven regular retinular cells contribute sets of alternately orthogonal microvilli to each band (Figs. 5, 6, 12). This unorthodox pattern should reduce polarization sensitivity and enhance sensitivity to unpolarized light.
-
3.
In this special region microvillus layers are strongly elliptical in cross section with the minor axis parallel to the microvilli (Fig. 12). Hence the ends of the major axes protrude considerably from the central area of overlap (Fig. 6).
-
4.
Retinular cell eight has bidirectional microvilli (Figs. 5–7) as usual in brachyuran crabs. Unlike the latter as well as paguroid crabs, Petrolisthes has square facets and a rectangular retinular array (Figs. 1, 3) similar to other galatheids and macruran decapods generally. It also resembles macrurans (shrimps and lobsters) in having perirhabdomal vacuoles absent or much reduced.
-
5.
Tight junctions occur widely between adjacent retinular cells (Figs. 14, 17) especially basally immediately distal to longitudinal zonular adherentes (Figs. 6, 16) typical of compound eyes. Freeze fracture reveals in addition numerous rectangular arrays of particles on the protoplasmic face of retinular cell membrane near, but not part of, the rhabdom (Figs. 19, 20). Other authors have hypothesized polarized transfer functions for similar particle aggregates in certain vertebrate cells.
-
6.
Experiments and further comparative study are needed to test whether the special fine structural features of the eye depend on Petrolisthes' sedentary photophobic habits or on its particular anomuran affiliation.
Similar content being viewed by others
References
Bennett MVL (1978) Junctional permeability. In: Feldman J, Gilula NB, Pitts JD (eds) Intercellular junctions and synapses: Receptors and recognition, Ser B, Vol 2. Chapman and Hall, London, pp 25–36
Bennett MVL, Goodenough DA (1978) Gap junctions, electrotonic coupling, and intercellular communication. Neurosciences Res Prog Bull 16:373–486
Bernhard CG (ed) (1966) The functional organization of the compound eye. Pergamon Press, Oxford, 591 pp
Bullock TH, Horridge GA (1965) Structure and function in the nervous system of invertebrates, Vol II. Freeman, San Francisco, London, pp 1068–1076
Chi C, Carlson SD (1980a) Membrane specializations in the first optic neuropil of the housefly, Musca domestica L. I. Junctions between neurons. J Neurocytol 9:429–449
Chi C, Carlson SD (1980b) Membrane specializations in the first optic neuropil of the housefly, Musca domestica L. II. Junctions between glial cells. J Neurocytol 9:451–469
Chi C, Carlson SD (1981a) Lanthanum and freeze fracture studies on the retinular cell junction in the compound eye of the housefly. Cell Tissue Res 214:541–552
Chi C, Carlson SD (1981b) The perineurium of the adult housefly: ultrastructure and permeability to lanthanum. Cell Tissue Res 217:373–386
Chi C, Carlson SD, St. Marie RL (1979) Membrane specializations in the peripheral retina of the housefly Musca domestica L. Cell Tissue Res 198:501–520
Coles JA, Tsacopoulos M (1979) Potassium activity in photoreceptors, glial cells and extracellular space in the drone retina: changes during photostimulation. J Physiol 290:525–549
Cummins D, Goldsmith TH (1981) Cellular identification of the violet receptor in the crayfish eye. J Comp Physiol 142:199–202
Deurs B van, Luft JH (1979) Effects of glutaraldehyde fixation on the structure of tight junctions. J Ultrastruct Res 68:160–172
Eguchi E (1978) Comparative fine structure of lepidopteran compound eyes, especially skippers (Hesperioidea). Zool Mag 87:32–43
Eguchi E (1982) Retinular fine structure in compound eyes of diurnal and nocturnal sphingid moths. Cell Tissue Res: in press
Eguchi E, Waterman TH (1966) Fine structure patterns in crustacean rhabdoms. In: Bernhard CG (ed) The functional organization of the compound eye. Pergamon Press, Oxford, pp 105–124
Eguchi E, Waterman TH (1967) Changes in retinal fine structure induced in the crab Libinia by light and dark adaptation. Z Zellforsch 79:209–229
Eguchi E, Waterman TH (1968) Cellular basis for polarized light perception in the spider crab, Libinia. Z Zellforsch 84:87–101
Eguchi E, Waterman TH (1973) Orthogonal microvillus pattern in the eighth rhabdomere of the rock crab Grapsus. Z Zellforsch 137:145–157
Eguchi E, Waterman TH (1976) Freeze-etch and histochemical evidence for cycling in crayfish photoreceptor membranes. Cell Tissue Res 169:419–434
Eley S, Shelton PMJ (1976) Cell junctions in the developing compound eye of the desert locust Schistocerca gregaria. J Embryol Exp Morphol 36:409–423
Ellisman MH, Brooke MH, Kaiser KK, Rash JE (1978) Appearance in slow muscle sarcolemma of specializations characteristic of fast muscle after reinnervation by a fast muscle nerve. Exp Neurol 58:59–67
Farges G (1975) Anatomie comparée du système neurosécréteur protocérébral chez cinq anomoures. Bull Soc Zool France 100:477–487
Fernández HR, Nickel EE (1976) Ultrastructrual and molecular characterisitics of crayfish photoreceptor membranes. J Cell Biol 69:721–732
Fincham AA (1980) Eyes and classification of malacostracan crustaceans. Nature 287:729–731
Goldsmith TH, Bernard GD (1974) The visual system of insects. In: Rockstein M (ed) The physiology of the insecta, 2nd Ed. Academic, New York San Francisco London, pp 165–262
Goodenough DA, Gilula NG (1979) Functional membrane differentiation at sites of cell-cell interaction. In: Nicholls JG (ed) The role of intercellular signals: Navigation, encounter, outcome. Dahlem Konferenzen, Berlin 1979, pp 135–154
Graf F (1978) Diversité structurale des jonctions intercellulaires communicantes (gap junctions) de l'épithélium des caecums postérieurs du Crustacé Orchestia. CR Acad Sc Paris 287:41–44
Gribakin FC (1979) Cellular mechanisms of insect photoreception. Int Rev Cytol 57:127–184
Hanström B (1948) The brain, the sense organs, and the incretory organs of the head in the Crustacea Malacostraca. Bull Biol France et Belgique, Suppl XXXIII, pp 98–126
Hatton JD, Ellisman MH (1981) The distribution of orthogonal arrays and their relationship to intercellular junctions in neuroglia of the freeze-fractured hypothalamo-neurohypophysial system. Cell Tissue Res 215:309–323
Horridge GA (ed) (1975) The compound eye and vision of insects. Clarendon Press, Oxford, 595 pp
Järvilehto M (1979) Receptor potentials in invertebrate visual cells. In: Autrum H (ed) Handbook of sensory physiology, Vol VII/6A. Springer, Berlin Heidelberg New York, pp 315–356
Krebs W (1972) The fine structure of the retinula of the compound eye of Astacus fluviatilis. Z Zellforsch 133:399–414
Kunze P (1979) Apposition and superposition eyes. In: Autrum H (ed) Handbook of sensory physiology, Vol VII/6A. Springer, Berlin Heidelberg New York, pp 441–502
Kurup NG (1964) The incretory organs of the eyestalk and brain of the porcelain crab, Petrolisthes cinctipes Randall (Reptantia-Anomura). Gen Comp Endocrinol 4:99–112
Land MF (1976) Superposition images are formed by reflection in the eyes of some oceanic decapod Crustacea. Nature 263:764–765
Land MF (1981) Optics and vision in invertebrates. In: Autrum H (ed) Handbook of sensory physiology, Vol VII/6B. Springer, Berlin Heidelberg New York, pp 471–592
Landis DMD, Reese TS (1974) Arrays of particles in freeze-fractured astrocytic membranes. J Cell Biol 60:316–320
Landis DMD, Reese TS (1981) Astrocyte membrane structure: changes after circulatory arrest. J Cell Biol 88:660–663
Lane NJ (1978) Electron microscopy. In: Sturgess JM (ed) State of the art symposia, vol 3. Proc 9th Internatl Congress on Electron Microscopy. The Imperial Press, Ltd, Canada, pp 673–691
Lane NJ (1981) Vertebrate-like tight junctions in the insect eye. Exp Cell Res 132:482–488
Lane NJ, Chandler HJ (1980) Definitive evidence for the existence of tight junctions in invertebrates. J Cell Biol 86:765–774
Lane NJ, Swales LS (1980) Dispersal of junctional particles, not internalization, during the in vivo disappearance of gap junctions. Cell 19:579–586
Lane NJ, Treherne JE (1980) Functional organisation of arthropod neuroglia. In: Locke M, Smith DS (eds) Insect biology in the future. Academic, New York, pp 765–795
Laughlin SD (1981) Neural principles in the peripheral visual system of invertebrates. In: Autrum H (ed) Handbook of sensory physiology, Vol VII/6B. Springer, Berlin Heidelberg New York, pp 133–280
Loewenstein WR (1978) Cell-to-cell communication. In: Andreoli TE, Hoffman JF, Fanestil DD (eds) Physiology of membrane disorders. Plenum, New York, pp 335–356
Menco BPhM (1980) Qualitative and quantitative freeze-fracture studies on olfactory and nasal respiratory epithelial surfaces of frog, ox, rat, and dog. III. Tight junctions. Cell Tissue Res 211:361–373
Meyer-Rochow V (1975) Larval and adult eye of the western rock lobster, Panulirus longipes. Cell Tissue Res 162:439–457
Meyer-Rochow V, Walsh S (1979) The eyes of mesopelagic crustaceans: I. Gennadas sp. (Penaeidae). Cell Tissue Res 184:87–101
Mote MI (1974) Polarization sensitivity. A phenomenon independent of stimulus intensity or state of adaptation in retinular cells of the crabs Carcinus and Callinectes. J Comp Physiol 90:389–403
Nässel DR (1976) The fine structure of photoreceptor terminals in the compound eye of Pandalus (Crustacea). Acta Zool 57:153–160
Nässel DR, Waterman TH (1977) Golgi EM evidence for visual information channelling in the crayfish lamina ganglionaris. Brain Res 130:556–563
Nässel DR, Waterman TH (1979) Massive diurnally modulated photoreceptor membrane turnover in crab light and dark adaptation. J Comp Physiol 131:205–216
Noirot-Timothée C, Noirot C (1980) Septate and scalariform junctions in arthropods. Int Rev Cytol 63:97–140
Overton J (1974) Cell junctions and their development. In: Cadenhead DA, Danielli JF, Rosenberg MD (eds) Progress in surface and membrane science, Vol 8. Academic Press, New York, pp 161–208
Parker GH (1981) The compound eyes in crustaceans. Bull Mus Comp Zool (Harvard) 21:45–140
Peracchia C (1974) Excitable membrane ultrastructure I. Freeze fracture of crayfish axons. J Cell Biol 61:107–122
Peracchia C (1980) Structural correlates of gap junction permeation. Int Rev Cytol 66:81–146
Ramadan MM (1952) Contribution to our knowledge of the structure of the compound eyes of Decapoda Crustacea. Lunds Univ Arsskrift 48:1–20
Revel JP, Karnvsky MJ (1967) Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol 33:C7-C12
Ribi WA (1978) Gap juctions coupling photoreceptor axons in the first optic ganglion of the fly. Cell Tissue Res 195:299–308
Rodieck RW (1973) The vertebrate retina. Freeman, San Francisco, 1044 pp
Rutherford DJ, Horridge GA (1965) The rhabdom of the lobster eye. QJ Microsc Sci 106:119–130
Sandri C, Akert K, Bennett MVL (1978) Junctional complexes and variations in gap junctions between spinal cord ependymal cells of a teleost, Sternarchus albifrons (Gymnotoidei). Brain Res 143:27–41
Schiller A, Forssmann WG, Taugner R (1980) The tight junctions of renal tubules in the cortex and outer medulla. Cell Tissue Res 212:395–413
Shaw SR (1966) Polarized light responses from crab retinula cells. Nature 211:92–93
Shaw SR (1978) The extracellular space and blood-eye barrier in an insect retina: an ultrastructural study. Cell Tissue Res 188:35–61
Shaw SR (1981) Anatomy and physiology of identified non-spiking cells in the photoreceptor-lamina complex of the compound eye of insects, especially Diptera. In: Roberts A, Bush BMH (eds) Neurones without impulses. Cambridge University Press, Cambridge, pp 61–116
Snyder AW (1979) Physics of vision in compound eyes. In: Autrum H (ed) Handbook of sensory physiology, Vol VII/6A. Springer, Berlin Heidelberg New York, pp 225–313
Staehelin LA (1974) Structure and function of intercellular junctions. Int Rev Cytol 39:191–283
Števčić Z (1971) The main features of brachyuran evolution. Syst Zool 20:331–340
Stowe S (1980a) Spectral sensitivity and retinal pigment movements in the crab, Leptograpsus variegatus (Fabricius). J Exp Biol 87:73–98
Stowe S (1980b) Rapid synthesis of photoreceptor membrane and assembly of new microvilli in a crab at dusk. Cell Tissue Res 211:419–440
Strausfeld NJ, Nässel DR (1981) Neuroarchitecture serving compound eyes of Crustacea and insects. In: Autrum H (ed) Handbook of sensory physiology, Vol VII/6B. Springer, Berlin Heidelberg New York, pp 1–132
Toh Y, Waterman TH (1982) Diurnal changes in compound eye fine structure in the blue crab Callinectes 1. Differences between noon and midnight retinas on an LD 11∶13 cycle. J Ultrastruct Res, 75: in press
Trujillo-Cenóz O (1972) The structural organization of the compound eye in insects. In: Fuortes MGF (ed) Handbook of sensory physiology, Vol VII/2. Springer, Berlin Heidelberg New York, pp 5–62
Vaughn JC, Traeger FJ (1976) Conservation of repeated DNA base sequences in the Crustacea: a molecular approach to decapod phylogeny. J Mol Evol 7:111–131
Vogt K (1975) Zur Optik des Flußkrebsauges. Z Naturforsch 30c:691
Vogt K (1977) Ray path and reflection mechanisms in crayfish eyes. Z Naturforsch 32c:466–468
Vogt K (1980) Die Spiegeloptik des Flußkrebsauges. J Comp Physiol 135:1–9
Waterman TH (1961) Light sensitivity and vision. In: Waterman TH (ed) The physiology of Crustacea, Vol II. Academic, New York, pp 1–64
Waterman TH (1966) Information channeling in the crustacean retina. In: Nye PW (ed) Proceedings of the symposium on information processing in sight sensory systems, 1965. California Institute of Technology, Pasadena, pp 48–56
Waterman TH (1975) The optics of polarization sensitivity. In: Snyder AW, Menzel R (eds) Photoreceptor optics. Springer, Berlin Heidelberg New York, pp 339–371
Waterman TH (1977) The bridge between visual input and central programming in crustaceans. In: Hoyle G (ed) Identified neurons and behavior of arthropods. Plenum, New York London, pp 371–386
Waterman TH (1981) Polarization sensitivity. In: Autrum H (ed) Handbook of sensory physiology, Vol VII/6B. Springer, Berlin Heidelberg New York, pp 281–469
Waterman TH (1982) Fine structure and turnover of photoreceptor membranes. In: Westfall JA (ed) Visual cells in evolution. Raven Press, New York, pp 23–42
Waterman TH, Fernández HR (1970) E-vector and wavelength discrimination by retinular cells of the crayfish Procambarus. Z Vergl Physiol 68:154–174
Waterman TH, Horch KW (1966) Mechanism of polarized light perception. Science 154:467–475
Waterman TH, Piekos WB (1981) Light and time correlated migration of invasive hemocytes in the crayfish compound eye. J Exp Zool 217:1–14
Waterman TH, Fernandez HR, Goldsmith TH (1969) Dichroism of photosensitive pigment in rhabdoms of the crayfish Orconectes. J Gen Physiol 54:415–432
Williams EH, DeHaan RL (1981) Electrical coupling among heart cells in the absence of ultrastructurally defined gap junctions. J Membr Biol 60:237–248
Williamson DI (1974) Larval characters and the origin of crabs (Crustacea, Decapoda, Brachyura). Thalassia Jugoslavica 10:401–414
Zampighi G, Corless JM, Robertson JD (1980) On gap junction structure. J Cell Biol 86:190–198
Author information
Authors and Affiliations
Additional information
Supported by grants from the Ministry of Education, Science and Culture of Japan (Special Project Research in Photophysiology, No. 411204, E.E., Principal Investigator) and from the U.S. National Institutes of Health (EY02929, T.H.W., Principal Investigator)
Rights and permissions
About this article
Cite this article
Eguchi, E., Goto, T. & Waterman, T.H. Unorthodox pattern of microvilli and intercellular junctions in regular retinular cells of the porcellanid crab Petrolisthes . Cell Tissue Res. 222, 493–513 (1982). https://doi.org/10.1007/BF00213850
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00213850