Abstract
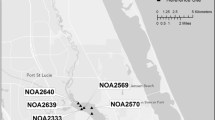
A chlor-alkali plant in Brunswick, Georgia, USA, discharged >2 kg mercury/d into a tributary of the Turtle River-Brunswick Estuary from 1966 to 1971. Mercury concentrations in sediments collected in 1989 along the tributary near the chlor-alkali plant ranged from 1 to 27 μg/g (dry weight), with the highest concentrations found in surface (0–8 cm) sediments of subtidal zones in the vicinity of the discharge site. Toxicity screening in 1990 using Microtox® bioassays on pore water extracted on site from sediments collected at six stations distributed along the tributary indicated that pore water was highly toxic near the plant discharge. Ten-day toxicity tests on pore water from subsequent sediment samples collected near the plant discharge confirmed high toxicity to Hyalella azteca, and feeding activity was significantly reduced in whole-sediment tests. In addition to mercury in the sediments, other metals (chromium, lead, and zinc) exceeded 50 μg/g, and polychlorobiphenyl (PCB) concentrations ranged from 67 to 95 μg/g. On a molar basis, acid-volatile sulfide concentrations (20–45 μmol/g) in the sediments exceeded the metal concentrations. Because acid-volatile sulfides bind with cationic metals and form metal sulfides, which are generally not bioavailable, toxicities shown by these sediments were attributed to the high concentrations of PCBs and possibly methylmercury.
Similar content being viewed by others
References
Allen HE, Gongmin F, Boothman W, Ditoro D, Mahony JD (1991) Draft analytical method for determination of acid volatile sulfide in sediment. US Environmental Protection Agency, Washington, DC
Arthur JW, West CW, Allen RN, Hedtke SF (1987) Seasonal toxicity of ammonia to five fish and nine invertebrate species. Bull Environ Contam Toxicol 38:324–331
Beitinger TL (1990) Behavioral reactions for the assessment of stress in fishes. J Great Lakes Res 16:495–528
Callister SM, Winfrey MR (1986) Microbial methylation of mercury in upper Wisconsin River sediments. Water Air Soil Pollut 29:453–465
Crane M, Maltby L (1991) The lethal and sublethal responses of Gammarus pulex to stress: Sensitivity and sources of variation in an in situ bioassay. Environ Toxicol Chem 10:1331–1340
Cripe GM, Ingley-Guezou A, Goodman LR, Forester J (1989) Effect of food availability on the acute toxicity of four chemicals to Mysidopsis bahia (Mysidacea) in static exposures. Environ Toxicol Chem 8:333–338
DiToro DM, Mahony JD, Hansen DJ, Scott KJ, Hicks MB, Mayr SM, Redmond MS (1990) Toxicity of cadmium in sediments: The role of acid volatile sulfide. Environ Toxicol Chem 9:1487–1502
Eisler R (1986) Polychlorinated biphenyl hazards to fish, wildlife, and invertebrates: A synoptic review. US Fish Wildl Serv Biol Rep 85(1.7)
Emerson K, Russo RC, Lund RE, Thurston RV (1975) Aqueous ammonia equilibrium: Effect of pH and temperature. J Fish Res Board Can 32:2379–2383
Fisher JB, Petty RL, Lick W (1983) Release of polychlorinated biphenyls from contaminated lake sediments: flux and apparent diffusivities of four individual PCBs. Environ Pollut Ser B 5:121–132
Gardner WS, Kendall DR, Odom RR, Windom HL, Stephens JA (1978) The distribution of methyl mercury in a contaminated salt marsh ecosystem. Environ Pollut 15:243–251
Gschwend PM, Wu SC (1985) On the constancy of sediment-water partition coefficients of hydrophobic organic pollutants. Environ Sci Technol 19:909–96
Hildebrand SG, Strand RH, Huckebee JW (1980) Mercury accumulation in fish and invertebrates of the North Fork Holston River, Virginia and Tennessee. J Environ Qual 9:393–400
Kenaga EE (1980) Predicted bioconcentration factors and soil sorption coefficients of pesticides and other chemicals. Ecotoxicol Environ Safety 4:26–38
Little EE, Flerov BA, Ruzhinskaya NN (1985) Behavioral approaches in aquatic toxicity investigations: A review. In: Mehrle PM, Gray RH, Kendall RL (eds) Toxic substances in the aquatic environment: A international aspect. Am Fish Soc, Water Quality Section, Bethesda, MD, pp 72–98
Luoma SN, Phillips DJH (1988) Distribution, variability, and impacts of trace elements in San Francisco Bay. Mar Pollut Bull 19:413–425
McLaren P (1982) Hydraulic control of grain-size distributions in a macro-tidal estuary. Sedimentology 29:437–439
Microbics Corporation (1990) A Microtox® manual—how to run toxicity tests using the Microtox® model 500. Carlsbad, CA
Mudroch A, Duncan GA (1986) Distribution of metals in different size fractions of sediment from the Niagara River. J Great Lakes Res 12:117–126
Odom RR (1974) Mercury contamination in Georgia rails. Proc Annu Conf Southeast Assoc Game Fish Comm 28:649–658
SAS Institute Inc (1985) SAS user's guide: Statistics, Version 5, Cary, NC
Safe S (1984) Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): Biochemistry, toxicology, and mechanisms of action. CRC Crit Rev Toxicol 13:319–393
Spry DJ, Wiener JG (1991) Metal bioavailability and toxicity to fish in low-alkalinity lakes: A critical review. Environ Pollut 71:243–304
Stalling D, Mayer FL (1972) Toxicities of PCBs to fish and environmental residues. Environ Health Perspect 1:159–164
Talbot V (1990) Mercury levels in biota and sediments of Princess Royal Harbour, Albany Western Australia: Interpretation and management implications. J Coast Res 6:545–558
Wade TL, Atlas EL, Brooks JM, Kennicutt MC II, Fox RG, Sericano J, Garcia B, Defreitas D (1988) NOAA Gulf of Mexico status and trends program: Trace organic contaminant distribution in sediments and oysters. Estuaries 11:171–179
Williams KA, Green DWJ, Pascoe D (1986) Studies on the acute toxicity of pollutants to freshwater macroinvertebrates. 3. Ammonia. Arch Hydrobiol 106:61–70
Windom H, Gardner W, Stephens J, Taylor F (1976) The role of methylmercury production in the transfer of mercury in a salt marsh ecosystem. Estuar Coast Mar Sci 4:579–583
Winger PV, Lasier PJ (1991) A vacuum-operated pore-water extractor for estuarine and freshwater sediments. Arch Environ Contam Toxicol 21:321–324
--, --(in press) Sediment toxicity testing: Comparison of methods and evaluation of influencing factors. In: Gorsuch JW, Dwyer FJ, Ingersoll CG, LaPoint TW (eds) Environmental toxicology and risk assessment: 2nd volume, ASTM STP 1173. American Society for Testing and Materials, Philadelphia, PA
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Winger, P.V., Lasier, P.J. & Geitner, H. Toxicity of sediments and pore water from Brunswick Estuary, Georgia. Arch. Environ. Contam. Toxicol. 25, 371–376 (1993). https://doi.org/10.1007/BF00210729
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00210729