Summary
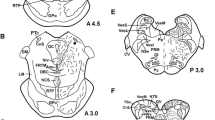
Noradrenaline has been shown to play an important role within the visual system of the brain. To analyze the postnatal development of alpha2-noradrenergic receptors in the visual system of tree shrews, we localized and quantified binding sites for the antagonist [3H]-rauwolscine by in vitro-autoradiography in the dorsal lateral geniculate nucleus and the striate cortex at different postnatal ages. At birth, the dorsal lateral geniculate nucleus is only slightly labeled by [3H]-rauwolscine. During the postnatal period, the number of binding sites increases to reach a maximum around postnatal day 20. Since the young tree shrews open their eyes at approximately day 19, it appears that this high concentration of alpha2-adrenoceptors is related to eye opening. In the adult animal, [3H]-rauwolscine labeling shows a laminated pattern in the dorsal lateral geniculate nucleus. Laminae 1, 2, and 3 are more strongly labeled than laminae 4, 5, and 6. In the striate cortex, the pattern of [3H]-rauwolscine-binding sites changes dramatically during the early postnatal period. Immediately after birth, there is only one layer, located within the subplate zone, which is labeled. From postnatal day 5 onwards, all cortical layers which can be distinguished on histologically stained sections reveal [3H]-rauwolscine-binding sites, but in layer IV, which is known to receive major inputs from the dorsal lateral geniculate nucleus, there is very little labeling during the first two postnatal weeks. In this layer, a large number of [3H]-rauwolscine-binding sites occurs between postnatal day 15 and 20, that is slightly before and around the time of eye opening. From this time onwards, the pattern of [3H]-rauwolscine binding in the striate cortex is very similar to that in the adult, where all layers are labeled although to different degrees. Since around postnatal day 20, maximal numbers of [3H]-rauwolscine binding sites are present in the dorsal lateral geniculate nucleus and a large number of these binding sites also emerges in layer IV of the striate cortex, alpha2-noradrenergic receptors are probably important for processes related to the opening of the eye and/or for the visual system to function.
Similar content being viewed by others
References
Arbilla S, Langer SZ (1990) The regulation of neurotransmitter release by alpha2-adrenoceptors in the central nervous system. In: Heal DJ, Marsden CA (eds) The pharmacology of noradrenaline in the central nervous system. Oxford University Press, Oxford, pp 141–154
Bear M, Singer W (1986) Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature 320:172–176
Bröcher S, Artola A, Singer W (1992) Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res 573:27–36
Brunso-Bechtold JK, Casagrande VA (1982) Early postnatal development of laminar characteristics in the dorsal lateral geniculate nucleus of the tree shrew. J Neurosci 2:589–597
Conley M, Fitzpatrick D, Diamond IT (1984) The laminar organization of the lateral geniculate body and the striate cortex in the tree shrew (Tupaia glis). J Neurosci 4:171–197
Conway JL, Schiller PH (1983) Laminar organization of tree shrew dorsal lateral geniculate nucleus. J Neurophysiol 50:1330–1342
Dermon CR, Kouvelas ED (1988) Binding properties, regional ontogeny and localization of adrenergic receptors in chick brain. Int J Dev Neuroscience 6:471–482
Diamond IT, Conley M, Fitzpatrick D, Raczkowski D (1991) Evidence for separate pathways within the tecto-geniculate projection in the tree shrew. Proc Natl Acad Sci USA 88:1315–1319
Elmslie KS, Cohen DH (1990) Iontophoresis of norepinephrine onto neurons of the pigeon's lateral geniculate nucleus: characterization of an inhibitory response. Brain Res 517:134–142
Flügge G, Jurdzinski A, Brandt S, Fuchs E (1990) Alpha2-adrenergic-binding sites in the medulla oblongata of tree shrews demonstrated by in vitro autoradiography: species-related differences in comparison to the rat. J Comp Neurol 297:253–266
Foelix RF, Kretz R, Rager G (1987) Structure and postnatal development of photoreceptors and their synapses in the retina of the tree shrew (Tupaia belangeri). Cell Tissue Res 247:287–297
Gallyas F (1979) Silver staining of myelin by means of physical development. Neurol Res 1:203–209
Geary WA, Toga AW, Woolen GF (1985) Quantitative film autoradiography for tritium: methodological considerations. Brain Res 337:99–108
Harting JK, Huerta MF, Hashikawa T, Van Lieshout DP (1991) Projection of the mammalian superior colliculus upon the dorsal lateral geniculate nucleus: organization of tectogeniculate pathways in nineteen species. J Comp Neurol 304:275–306
Hashikawa T, Van Lieshout D, Harting JK (1986) Projections from the parabigeminal nucleus to the dorsal lateral geniculate nucleus in the tree shrew Tupaia glis. J Comp Neurol 246:382–394
Hubel DH (1975) An autoradiographic study of the retino-cortical projections in the tree shrew (Tupaia glis). Brain Res 96:41–50
Kostovic I, Rakic P (1990) Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol 297:441–470
Kretz R, Rager G, Norton TT (1986) Laminar organization of ON and OFF regions and ocular dominance in the striate cortex of the tree shrew (Tupaia belangeri). J Comp Neurol 251:135–145
Kretz R, Saini K, Rager G (1987) On and Off-channels in the geniculostriate pathway of Tupaia: a single unit study. Acta Anat 128:341
Kromer LF, Moore RY (1980) A study of the organization of the locus coeruleus projections to the lateral geniculate nuclei in the albino rat. Neuroscience 5:255–271
Kuhar MJ, Unnerstall JR (1985) Quantitative receptor mapping by autoradiography: some current technical problems. Trends NeuroSci 8:49–53
Levitt P, Moore RV (1979) Origin and organization of brainstem catecholamine innervation in the rat. J Comp Neurol 186:505–528
Liu YL, Jia WG, Strosberg AD, Cynader M (1992) Morphology and distribution of neurons and glial cells expressing beta-adrenergic receptors in developing kitten visual cortex. Dev Brain Res 65:269–273
Lund JS, Fitzpatrick D, Humphrey AL (1985) In: Peters A, Jones EG (eds) The striate visual cortex of the tree shrew. Cerebral cortex, vol 3. Plenum Press, New York, pp 157–205
Perry BD, U'Prichard DC (1984) Alpha-adrenergic receptors in neural tissues: methods and applications of radioligand binding assays. In: Marangos PJ, Campbell IC, Cohen RM (eds) Brain receptor methodologies, Part A. Academic Press, New York, pp 255–283
Petrash AC, Bylund DB (1986) Alpha2-adrenergic receptor sub-types indicated by [3H]-yohimbine in the human brain. Life Sci 38:2129–2137
Rager G (1991) The visual cortex of Tupaia — an alternative model. J Hirnforsch 32:537–540
Rakic P, Goldman-Rakic PS, Gallager D (1988) Quantitative auto-radiography of major neurotransmitter receptors in the monkey striate and extrastriate cortex. J Neurosci 8:3670–3690
Rosenthal HE (1967) A graphic method for the determination and presentation of binding parameters in a complex system. Anal Biochem 20:525–532
Saini K, Kretz R, Rager G (1987) Classes of neurons in relation to the laminar organization of the lateral geniculate nucleus in the tree shrew, Tupaia belangeri. J Comp Neurol 259:31–49
Shaw C, Cynader M (1986) Laminar distribution of receptors in monkey (Macaca fascicularis) geniculate system. J Comp Neurol 248:301–312
Specht LA, Pickel VM, Tong HJ, Reis DJ (1981) Light-microscopic immunocytochemical localization of tyrosine hydroxylase in prenatal rat brain. II. Late ontogeny. J Comp Neurol 199:255–276
Starke K (1987) Presynaptic alpha-autoreceptors. Rev Physiol Biochem Pharmacol 107:73–146
Tigges J, Shanta TR (1969) A stereotaxic brain atlas of the tree shrew (Tupaia glis). Williams & Wilkins, Baltimore
Unnerstall JR, Kopajtic TA, Kuhar MJ (1984) Distribution of alpha2-agonist-binding sites in the rat and human central nervous system: analysis of some functional, anatomic correlates of the pharmacologic effects of clonidine and related adrenergic agents. Brain Res 7:69–101
Zilles KJ (1978) Ontogenesis of the visual system. Springer, Heidelberg
Zilles K, zur Nieden K, Schleicher A, Traber J (1990) A new method for quenching correction leads to revisions of data in receptor autoradiography. Histochemistry 94:569–578
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Flügge, G., Fuchs, E. & Kretz, R. Postnatal development of 3H-rauwolscine binding sites in the dorsal lateral geniculate nucleus and the striate cortex of the tree shrew (Tupaia belangeri). Anat Embryol 187, 99–106 (1993). https://doi.org/10.1007/BF00208200
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00208200