Summary
Ion-selective microelectrodes inserted into the compound eyes of Calliphora were used to monitor the changes in extracellular concentration of Ca2+ and Na+ (Cao, Nao) brought about by a 1-min exposure to white light (maximal luminous intensity ≈0.1 cd/cm2).
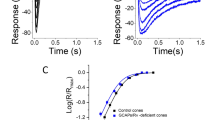
Using Ringer solution as the reference (Ca2+ = 1 mM), the dark concentration of the calcium in the retina was found to be (1.4 ± 0.4) mM (n=12). Stimulation with light reduces Cao. At intensities near maximal the Cao signal is phasic, reaching a transient minimum about 6 s after light onset \((\Delta {\text{Ca}}_{\text{o}} {\text{ = }} - 6.28\% \pm 1.6\% ,{\text{ }}n = 20)\) and then rising to a nearly stable plateau below the dark level (-3.3% ± 2.6%). Cao signals measured in the white-eyed mutant (chalky), which lacks pigment granules, are comparable to those in the wild type.
Conclusions: (a) There are no extracellular Ca2+ binding sites that regulate light adaptation, such as were postulated by Hochstrate and Hamdorf (1985). (b) Ca2+ influx into the photoreceptors seems to be necessary for light adaptation, (c) The pigment granules have no major function in intracellular calcium regulation.
The time course of the Nao signals resembles that of the Cao signals. Because the percentage concentration change is small, light-induced extracellular Na+-depletion cannot contribute to a reduced response amplitude at light adaptation.
Similar content being viewed by others
Abbreviations
- Ca i :
-
intracellular Ca2+ concentration
- Ca o :
-
extracellular Ca2+ concentration
- Kino :
-
extracellular K+ concentration
- Na o :
-
extracellular Na+ concentration
References
Ammann D (1986) Ion-selective microelectrodes: principles, design and application. Springer, Berlin Heidelberg New York
Ammann D, Chao P, Simon W (1987) Valinomycin-based K+ selective microelectrodes with low electrical membrane resistance. Neurosci Lett 74:221–226
Anton-Erxleben F (1989) X-ray microanalysis of screening pigment granula in the ommatidia of Drosophila melanogaster. Verh Dtsch Zool Ges 82:270
Baumann F (1968) Slow and spike potentials recorded from retinula cells of the honeybee drone in response to light. J Gen Physiol 52:855–875
Baumann O, Walz B (1989a) Topography of Ca2+-sequestering endoplasmic reticulum in photoreceptors and pigmented glial cells in the compound eye of the honeybee drone. Cell Tissue Res 255:511–522
Baumann O, Walz B (1989b) Calcium- and inositol polyphosphate-sensitivity of the calcium-sequestering endoplasmic reticulum in the photoreceptor cells of the honeybee drone. J Comp Physiol A 165:627–636
Baumann O, Walz B, Somlyo AV, Somlyo AP (1991) Electron probe microanalysis of calcium release and magnesium uptake by endoplasmic reticulum in bee photoreceptors. Proc Natl Acad Sci USA 88:741–744
Berridge MJ, Irvine RF (1989) Inositol phosphates and cell signalling. Nature 341:197–205
Blaustein MP (1988) Calcium transport and buffering in neurons. Trends Neurosci 11:438–443
Bolsover SR, Brown JE (1985) Calcium ion, an intracellular messenger of light adaptation, also participates in excitation of Limulus ventral photoreceptor cells. J Physiol (Lond) 364:389–393
Brown HM, Rydquist B, Moser H (1988) Intracellular calcium changes in Balanus photoreceptor. A study with calcium ionselective electrodes and arsenazo III. Cell Calcium 9:105–119
Brown JE (1986) Calcium and light adaptation in invertebrate photoreceptors. In: Stieve H (ed) The molecular mechanism of photoreception. Dahlem Konferenzen. Springer, Berlin Heidelberg New York, pp 231–240
Brown JE, Blinks JR (1974) Changes in intracellular free calcium concentration during illumination of invertebrate photoreceptors. Detection with aequorin. J Gen Physiol 64:643–665
Brown JE, Mote MI (1974) Ionic dependence of reversal voltage of the light response in Limulus ventral photoreceptors. J Gen Physiol 63:337–350
Carafoli E (1987) Intracellular calcium homeostasis. Ann Rev Biochem 56:395–433
Case R (1957) Differentiation of the effects of pH and CO2 on the spiracular function of insects. J Cell Comp Physiol 49:103–113
Chi C, Carlson SD (1981) Lanthanum and freeze fracture studies on the retinula cell junction in the compound eye of the housefly. Cell Tissue Res 214:541–552
Coles JA (1986) Homeostasis of extracellular fluid in retinas of invertebrates and vertebrates. Progress in Sensory Physiology 6. Springer, Berlin Heidelberg New York, pp 105–138
Coles JA, Orkand RK (1985) Changes in sodium activity during light stimulation in photoreceptors, glia and extracellular space in drone retina. J Physiol (Lond) 362:415–435
Coles JA, Schneider-Picard G (1989) Amplification of small signals by voltage-gated sodium channels in drone photoreceptors. J Comp Physiol A 165:109–118
Devary O, Heichal O, Blumenfeld A, Cassel D, Suss E, Barash S, Rubinstein CT, Minke B, Selinger Z (1987) Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc Natl Acad Sci USA 84:6939–6943
Dörrscheidt-Käfer M (1972) Die Empfindlichkeit einzelner Photorezeptoren im Komplexauge von Calliphora erythrocephala. J Comp Physiol 81:309–340
Fein A, Tsacopoulos M (1988) Activation of mitochondrial oxidative metabolism by calcium ions in Limulus ventral photoreceptor. Nature 331:437–440
Franceschini N, Kirschfeld K (1976) Le controle automatique du flux lumineux dans l'oeil composé des diptères. Propriétés spectrales, statiques et dynamiques du mécanisme. Biol Cybern 21:181–203
Gorman ALF, Levy S, Nasi E, Tillotson D (1984) Intracellular calcium measured with calcium-sensitive micro-electrodes and arsenazo III in voltage-clamped Aplysia neurones. J Physiol (Lond) 353:127–142
Hamdorf K, Schwemer J (1975) Photoregeneration and the adaptation process in insect photoreceptors. In: Snyder AW, Menzel R (eds) Photoreceptor optics. Springer, Berlin Heidelerg New York, pp 363–389
Hansen AJ (1985) Effect of anoxia on ion distribution in the brain. Physiol Rev 65:101–148
Harary HH, Brown JE (1984) Spatially nonuniform changes in intracellular calcium ion concentrations. Science 224:292–294
Hardie RC (1985) Functional organization of the fly retina. Progress in Sensory Physiology 5. Springer, Berlin Heidelberg New York, pp 1–79
Hausen K (1976) Struktur, Funktion und Konnektivität bewegungs-empfindlicher Interneuronen im dritten optischen Neuropil der Schmeißfliege Calliphora erythrocephala. Dissertation, Universität Tübingen
Hausen K (1982) Motion sensitive interneurons in the optomotor system of the fly I. The horizontal cells: structure and signals. Biol Cybern 45:143–156
Heinemann U, Lux HD, Gutnick MJ (1977) Extracellular free calcium and potassium during paroxysmal activity in cerebral cortex of the cat. Exp Brain Res 27:237–243
Hochstrate P (1989) Photoresponses from cells in the fly's eye which are not visual cells. Z Naturforsch 44c:867–875
Hochstrate P, Hamdorf K (1985) The influence of extracellular calcium on the response of fly photoreceptors. J Comp Physiol A 156:53–64
Hodgkin AL, McNaughton PA, Nunn BJ (1985) The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol (Lond) 358:447–468
Hofmeier G, Lux HD (1981) The time course of intracellular free calcium and related electrical effects after injection of CaCl2 into neurons of the snail, Helix pomatia. Pflügers Arch 391:242–251
Howard J (1984) Calcium enables photoreceptor pigment migration in a mutant fly. J Exp Biol 113:471–475
IUPAC Commission on Analytical Nomenclature, prepared for publication by Guilbault GG (1979) Recommendations for publishing manuscripts on ion-selective electrodes. Ion-Selective Electrode Rev 1:139–143
Ivens I, Stieve H (1984) Influence of the membrane potential on the intracellular light induced Ca2+-concentration change of the Limulus ventral photoreceptor monitored by arsenazo III under voltage clamp conditions. Z Naturforsch 39c:986–992
Jansonius NM (1990) Properties of the sodium pump in the blowfly photoreceptor cell. J Comp Physiol A 167:461–467
Kirschfeld K, Vogt R (1980) Calcium ions and pigment migration in fly photoreceptors. Naturwissenschaften 67:516–517
Langer H (1962) A new eye colour mutation in Calliphora erythrocephala Meig. Nature 194:111–112
Lanter F, Steiner RA, Ammann D, Simon W (1982) Critical evaluation of the applicability of neutral carrier-based calcium selective microelectrodes. Anal Chim Acta 135:51–59
Laughlin SB, Hardie RC (1978) Common strategies for light adaptation in the peripheral visual systems of the fly and dragonfly. J Comp Physiol 128:319–340
Levy S, Fein A (1985) Relationship between light sensitivity and intracellular free Ca concentration in Limulus ventral photoreceptors. A quantitative study using Ca-selective microelectrodes. J Gen Physiol 85:805–841
Lux HD, Neher E (1973) The equilibration time course of [K o+ ] in cat cortex. Exp Brain Res 17:190–205
Maaz G, Stieve H (1980) The correlation of the receptor potential with the light induced transient increase in intracellular calciumconcentration measured by absorption change of arsenazo III injected into Limulus ventral nerve photoreceptor cell. Biophys Struct Mech 6:191–208
Martin RL, Hafner GS (1986) Factors influencing the degradation of photoreceptor membrane in the crayfish, Procambarus clarkii. Cell Tissue Res 243:205–212
Matic T, Laughlin SB (1981) Changes in the intensity-response function of an insect's photoreceptor due to light adaptation. J Comp Physiol 145:169–177
Meier PC (1982) Two-parameter Debye-Hückel approximation for the evaluation of mean activity coefficients of 109 electrolytes. Anal Chim Acta 136:363–368
Minke B, Armon E (1984) Activation of electrogenic Na-Ca exchange by light in fly photoreceptors. Vision Res 24:109–115
Minke B, Tsacopoulos M (1986) Light induced sodium dependent accumulation of calcium and potassium in the extracellular space of bee retina. Vision Res 26:679–690
Muijser H (1979) The receptor potential of retinular cells of the blowfly Calliphora: the role of sodium, potassium and calcium ions. J Comp Physiol 132:87–95
O'Day PM, Lisman JE, Goldring M (1982) Functional significance of voltage-dependent conductances in Limulus ventral photoreceptors. J Gen Physiol 79:211–232
Oehme M, Simon W (1976) Microelectrode for potassium ions based on a neutral carrier and comparison of its characteristics with a cation exchanger sensor. Anal Chem 86:21–225
Oehme M, Kessler M, Simon W (1976) Neutral carrier Ca2+-microelectrode. Chimia 3:204–206
Orkand RK, Dietzel I, Coles JA (1984) Light-induced changes in extracellular volume in the retina of the drone, Apis mellifera. Neurosci Lett 45:273–278
Payne R (1986) Phototransduction by microvillar photoreceptors of invertebrates: mediation of a visual cascade by inositol trisphosphate. Photobiochem Photobiophys 86:373–397
Payne R, Walz B, Levy S, Fein A (1988) The localization of calcium release by inositol trisphosphate in Limulus photoreceptors and its control by negative feedback. Phil Trans R Soc Lond B 320:359–379
Pugh EN, Brown JE, Hamdorf K, Hillman P, Hochstrate P, Keiper WJM, Kirschfeld K, Lamb TD, Minke B, Pepperberg DR, Schwemer J, Shapley R (1986) Adaptation. Group Report. In: Stieve H (ed) The molecular mechanism of photoreception. Dahlem Konferenzen. Springer, Berlin Heidelberg New York, pp 467–488
Roebroek JGH, Stavenga DG (1990) Insect pupil mechanisms. IV. Spectral characteristics and light intensity dependence in the blowfly, Calliphora erythrocephala. J Comp Physiol A 166:537–543
Sandler C (1990a) Homeostasis of extracellular fluid in retinas of drones and flies. In: Elsner N, Roth G (eds) Brain — perception — cognition. Proceedings of the 18th Göttingen neurobiology conference. Thieme, Stuttgart New York, p 212
Sandler C (1990b) Ionenhomöostase in der Insektenretina — Calcium, Lichtadaptation und Neuron-Glia-Interaktion. Dissertation, Eberhard-Karls-Universität, Tübingen
Sandler C, Kirschfeld K (1988) Light intensity controls extracellular Ca2+ concentration in the blowfly retina. Naturwissenschaften 75:256–258
Sandler C, Kirschfeld K (1989) Photostimulation decreases the extracellular Ca2+ concentration in the blowfly retina and lamina. In: Elsner N, Singer W (eds) Dynamics and plasticity in neuronal systems. Proceedings of the 17th Göttingen neurobiolgy conference. Thieme, Stuttgart New York, p 90
Schröder W, Frings D, Stieve H (1980) Measuring calcium uptake and release by invertebrate photoreceptor cells by laser microprobe mass spectroscopy. Scanning Electron Microsc 11:647–656
Shaw SR (1978) The extracellular space and blood-eye barrier in an insect retina: an ultrastructural study. Cell Tissue Res 188:35–61
Tinbergen J, Stavenga DG (1987) Spectral sensitivity of light induced respiratory activity of photoreceptor mitochondria in the intact fly. J Comp Physiol A 160:195–203
Tsien RY, Rink TJ (1980) Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta 599:623–638
Tsuda M (1987) Photoreception and phototransduction in invertebrate photoreceptors. Photochem Photobiol 45:915–931
Walz B (1982) Calcium-sequestering smooth endoplasmic reticulum in retinula cells of the blowfly. J Ultrastruct Res 81:240–248
Weingart R, Hess P (1984) Free calcium in sheep cardiac tissue and frog skeletal muscle measured with Ca2+-selective microelectrodes. Pflügers Arch 402:1–9
White RH, Michaud NA (1980) Calcium is a component of ommochrome pigment granules in insect eyes. Comp Biochem Physiol 65A:239–242
Wuhrmann P, Ineichen H, Riesen-Willi U, Lezzi M (1979) Change in nuclear potassium electrochemical activity and puffing of potassium-sensitive salivary chromosome regions during Chironomus development. Proc Natl Acad Sci USA 76:806–808
Yau KW, Nakatani K (1984) Cation selectivity of light-sensitive conductance in retinal rods. Nature 309:352–354
Ziegler A, Walz B (1989) Analysis of extracellular calcium and volume changes in the compound eye of the honeybee drone, Apis mellifera. J Comp Physiol A 165:697–709
Ziegler A, Walz B (1990) Evidence for light-induced release of Ca2+ from intracellular stores in bee photoreceptors. Neurosci Lett 111:87–91
Ziegler I, Harmsen R (1969) The biology of pteridines in insects. Adv Insect Physiol 6:139–203
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sandler, C., Kirschfeld, K. Light-induced extracellular calcium and sodium concentration changes in the retina of Calliphora: involvement in the mechanism of light adaptation. J Comp Physiol A 169, 299–311 (1991). https://doi.org/10.1007/BF00206994
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00206994