Abstract
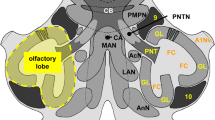
The distribution of GABA-immunoreactivity was studied in the brain of the silver eel (Anguilla anguilla) by means of antibodies directed against GABA. Immunoreactive neuronal somata were distributed throughout the brain. Positive perikarya were detected in the internal cellular layer of the olfactory bulb, and in all divisions of the telencephalon, the highest density being observed along the midline. Numerous GABA-reactive cell bodies were found in the diencephalon, particularly in the preoptic and tuberal regions of the hypothalamus, and the dorsolateral, dorsomedial and ventromedial thalamic nuclei. In the optic tectum, the majority of GABApositive cell bodies were located in the periventricular layer. A number of immunolabelled cell bodies were observed in different tegmental structures, notably the torus semicircularis. In the cerebellum, the Purkinje cells were either very intensely or very weakly immunoreactive. In the rhombencephalon, reactive cell bodies were observed in the eminentia granularis, the valvula cerebellaris, the octavolateral nucleus, the lobus vagus and in the vagal and glossopharyngeal motor nuclei. Intensely immunoreactive axons and terminals were observed in the external granular layer and internal cellular layer of the olfactory bulb. In the telencephalon, the highest density of reactive fibres and boutons was found in the fields of the medial wall. Many immunolabelled fibres were seen in the medial and lateral forebrain bundles. In the diencephalon, intense labelling of fibres and terminals were observed in the nuclei situated close to the midline. In the optic tectum the highest density of reactive fibres was seen in the sfgs, the layer to which the retina projects massively. Finally, in the rhombencephalon the strongest labelling of neurites was observed in the nuclei of the raphé, the nucleus octavocellularis magnocellularis and the nuclei of the IXth and Xth cranial nerves. The GABAergic system of the eel, which is well developed, appears to be generally comparable to that described in tetrapod vertebrates.
Similar content being viewed by others
References
Airhart MJ (1979) Telencephalotectal projections in the goldfish, Carassius auratus: a light and electron microscopic study. Anat Rec l93:468
Antal M (1991) Distribution of GABA immunoreactivity in the optic tectum of the frog: a light and electron microscopic study. Neuroscience 42:879–891
Augustine JR (1987) Immunocytochemical staining of GABA in the insular lobe of the savanna baboon: a light microscopic study. Brain Res 424:352–360
Ball JN (1981) Hypothalamic control of the pars distalis in fishes, amphibians and reptiles. Gen Comp Endocrinol 44:135–170
Bass AH (1989) Telencephalic efferents in the channel catfish, Ictalurus punctatus. Projections to the olfactory bulb and optic tectum. Brain Behav Evol 19:1–16
Belekhova MG, Kratskin IL, Repérant J, Pierre J, Vesselkin NP, Kenigfest NB, Tumanova NL, Chkheidze DD (1990) Localisation of GABA-immunoreactive elements in the thalamus of the tortoise Emys orbicularis (in Russian). J Evol Biokl Physiol 27:676–684
Belekhova MG, Chkeidze DD, Vesselkin NP, Kenigfest NB, Kratskin IL, Pierre J, Repérant J (1992) Study of GABA-immunoreactive elements distribution in amygdaloid complex of reptiles (in Russian). Neurophysiology (USSR) 24:68–76
Bennis M, Calas A, Geffard M, Gamrani H (1991) Distribution of GABA immunoreactive systems in the forebrain and midbrain of the chameleon. Brain Res Bull 26:891–898
Blanton MJ, Shen JM, Kriegstein AR (1987) Evidence for the inhibitory neurotransmitter gamma-aminobutyric acid in aspiny and sparsely spiny non-pyramidal neurons of the turtle dorsal cortex. J Comp Neurol 259:277–297
Chan-Palay V, Lin CT, Palay SL, Yamamoto M, Wu JY (1982) Taurine in the mammalian cerebellum. Demonstration by autoradiography with 3H taurine and immunochemistry with antibodies against the taurine-synthesizing enzyme, cysteine-sulfinic acid decarboxylase. Proc Natl Acad Sci USA 79:2695–2699
Clayton DF, Alvarez-Buylla A (1989) In situ hybridization using PEG-embedded tissue and riboprobes: increased cellular detail coupled with high sensitivity. J Histochem Cytochem 37:389–393
Contestable A, Villani L, Bissoli R, Poli A, Migan P (1986) Cholinergic, GABAergic and excitatory amino acidic neurotransmission in the goldfish vagal lobe. Exp Brain Res 63:301–309
Decavel C, Van den Pol AN (1990) GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol 302:1019–1037
Denizot P, Clause S, Elekes K, RavailleVeron M, Szabo T (1987) Convergence of electronic club endings of GABA-serotoninergic terminals on second-order neurons of the electrosensory pathway in mormyryd fish Gnathonemus petersii and Brienomyrus niger (Teleostei). Cell Tissue Res 249:301–309
DeRopp RS, Kastl LH, Furst A (1970) Comparative effects of temperature on brain enzymes in goldfish (Carassius auratus) and mouse (Mus musculus). Comp Biochem Physiol 37:123–125
Domenici L, Waldvogel HJ, Matute C, Streit P (1988) Distribution of GABA-like immunoreactivity in the pigeon brain. Neuroscience 25:931–950
Echteler SM (1984) Connections of the auditory midbrain in a teleost fish Cyprinus carpio. J Comp Neurol 230:536–551
Echteler SM (1985) Organization of central auditory pathways in a teleost fish (Cyprinus carpio). J Comp Physiol A 156:267–280
Echteler SM, Saidel WM (1981) Forebrain connections in the goldfish support telencephalic homologies with land vertebrates. Science 212:683–685
Ekström P (1987) Distribution of choline acetyltransferase-immunoreactive neurons in the brain of a cyprinid teleost (Phoxinus phoxinus L.) J Comp Neurol 256:494–515
Ekström P, Van Veen T, Bruum A, Ehing B (1987) GABA-immunoreactive neurons in the photosensory pineal organ of the rainbow trout: two distinct neuronal populations. Cell Tissue Res 250:87–92
Enna SJ, Gallagher JP (1983) Biochemical and electrophysiological characteristics of mammalian GABA receptors. Int Rev Neurobiol 24:121–181
Everitt BJ, Hökfelt T, Wu JY, Goldstein M (1984) Coexistence of tyrosine hydroxylase-like and gamma-aminobutyric acid-like immunoreactivities in neurons of arcuate nucleus. Neuroendocrinology 39:189–191
Franzoni MF, Morino P (1989) The distribution of GABA-like-immunoreactive neurons in the brain of the newt, Triturus cristatus carnifex and the green frog, Rana esculenta. Cell Tissue Res 255:155–166
Frember M, Van Veen TH, Hartwig GH (1977) Formaldehyde-induced fluorescence in the telencephalon and diencephalon of the eel (Anguilla anguilla L.). Cell Tissue Res 176:1–22
Fryer JN, Boudreault-Chateauvert C, Kirby RP (1985) Pituitary afferents originating in the paraventricular organ (PVO) of the goldfish hypothalamus. J Comp Neurol 212:475–484
Garey LJ, Takàcs J, Revishchin AV, Hàmori J (1989) Quantitative distribution of GABA-immunoreactive neurons in cetacean visual cortex is similar to that of land mammals. Brain Res 485:278–284
Ito H, Kishida R (1977) Tectal afferent neurons identified by the retrograde HRP method in the carp telencephalon. Brain Res 130:142–145
Ito H, Kishida R (1978) Telencephalic afferent neurons identified by the retrograde HRP method in the carp diencephalon. Brain Res 149:211–215
Ito H, Morita Y, Sakamoto N, Ueda S (1980) Possibility of telencephalic visual projection in teleosts, Holocentridae. Brain Res 197:219–222
Ito H, Murakami T, Fukuoka T, Kishida R (1986) Thalamic fiber connections in a teleost (Sebasticus marmoratus): visual somatosensory, octavolateral, and cerebellar relay region to telencephalon. J Comp Neurol 250:215–227
Johnston SA, Maler L (1992) Anatomical organisation of the hypophysiotrophic systems in the electric fish, Apteronotus leptorhynchus. J Comp Neurol 317:421–437
Kah O (1983) Approche morphofonctionelle des relations hypothalamo-hypophysaires chez les poissons téleostéens. Thèse Dr. Sci 768, Université de Bordeaux, pp 1–120
Kah O, Dubourb P, Chambolle P, Calas A (1984a) Ultrastructural identification of catecholaminergic fibers in the goldfish pituitary. Cell Tissue Res 238:621–626
Kah O, Chambolle P, Thibault J, Geffard M (1984b) Existence of dopaminergic neurons in the preoptic region of the goldfish. Neurosci Lett 48:293–298
Kah O, Dufour S, Baloche S, Breton B (1986 a) Immunocytochemical study of Gn-RH systems in the brain and pituitary of normal and hCG-treated European eels. Israeli symposium on reproduction in fish. Basic and applied aspects in endocrinology and genetics, Tel Aviv 1986. Ed INRA Paris 1988 Colloques de l'INRA44:81–84
Kah O, Breton B, Dulka JG, Nunez-Rodriguez J, Peter RE, Corrigan A, Rivier JE, Vale WW (1986b) A reinvestigation of the Gn-RH (gonadotrophin-releasing hormone) systems in the goldfish brain using antibodies to salmon Gn-RH. Cell Tissue Res 244:577–582
Kah O, Dubourg P, Onteniente B, Geffard M, Calas A (1986c) The dopaminergic innervation of the goldfish pituitary. Cell Tissue Res 224:577–582
Kah O, Dubourg P, Martinoli MG, Rabhi M, Gonnet F, Geffard M, Calas A (1987a) Central GABAergic innervation of the pituitary in goldfish: a radioautographic and immunocytochemical study at the electron microscope level. Gen Comp Endocrinol 67:324–332
Kah O, Dubourg P, Martinoli MG, Geffard M, Calas A (1987b) Morphological evidence for a direct GABAergic control of the anterior pituitary in teleosts. Experientia 43:300–302
Kah O, Dufour S, Baloche S, Breton B (1989) The GnRH systems in the brain and pituitary of normal and hCG treated european silver eel. Fish Physiol Biochem 6:279–284
Kah O, Trudeau VL, Sloley BD, Martinoli MG, Chang JP, Yu KL, Peter RE (1991) Implication of GABA in the neuroendocrine regulation of gonadotropin release in the goldfish (Carassius auratus). In: Scott AP, Sumpter JP, Kime DE, Rolfe MS (eds) Proceedings of the Fourth International Symposium on the Reproductive Physiology of Fish. Norwich, Fish Symp 91, Sheffield, pp 57–59
Kah O, Trudeau VL, Sloley BD, Chang JP, Dubourg P, Yu KL, Peter RE (1992) Influence of GABA on gonadotrophin release in the goldfish. Neuroendocrinology 55:396–404
Kaul S, Vollrath L (1974) The goldfish pituitary. II. Innervation. Cell Tissue Res 154:231–249
Kosaka Y, Tauchi M, Dabl JL (1988) Cholinergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res 70:605–617
Lauder GV, Liem KF (1983) The evolution and interrelationships of the actinopterygian fishes. Bull Mus Comp Zool 150:95–197
Leranth C, Maclusky MJ, Sakamoto H, Shanabrough M, Naftolin F (1985) Glutamic acid decarboxylase-containing axons synapse on LH-RH neurons in the rat medial preoptic area. Neuroendocrinology 40:536–539
Martinoli MG, Dubourg P, Geffard M, Calas A, Kah O (1990) Distribution of GABA-immunoreactive neurons in the forebrain of the goldfish, Carassius auratus. Cell Tissue Res 260:74–84
Médina M, Le Belle N, Repérant J, Rio JP, Ward R (1990) An experimental study of the retinal projections of the european eel (Anguilla anguilla) carried out at the catadromic migratory silver stage. J Hirnforsch 31:467–480
Meek J (1983) Functional anatomy of the tectum mesencephali of the goldfish. An explorative analysis of the functional implications of the laminar structural organization of the tectum. Brain Res Rev 6:247–297
Meek J, Schellart NAM (1978) A Golgi study of goldfish optic tectum. J Comp Neurol 182:89–122
Meek J, Joosten HWJ, Steinbusch HWM (1989) The distribution of dopamine-immunoreactivity in the brain of the mormyrid teleost Gnathonemus petersii. J Comp Neurol 282:362–383
Morita Y, Ito H, Masai H (1980) Central gustatory paths in the crucian carp, Carrassius carassius. J Comp Neurol 191:119–132
Mugnaini E, Maler L (1987) Cytology and immunohistochemistry of the nucleus extralateralis anterior of the mormyrid brain: possible role of GABAergic synapses in temporal analysis. Anat Embryol 176:313–326
Mugnaini E, Oertel WH (1985) An atlas of distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunochemistry. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy, vol 4, Part 1. Elsevier, Amsterdam, pp 436–622
Murakami T, Morita Y, Ito H (1983) Extrinsic and intrinsic fiber connections of the telencephalon in a teleost, Sebasticus marmoratus. J Comp Neurol 216:115–131
Nagai T, McGeer PL, McGeer EG (1983) Distribution of GABAT-intensive neurons in the rat forebrain and midbrain. J Comp Neurol 218:220–238
Nieuwenhuys R (1962a) The morphogenesis and the general structure of the actinopterygian forebrain. Acta Morphol Neerl Scand 5:65–78
Nieuwenhuys R (1962b) Trends in the evolution of the actinopterygian forebrain. J Morphol 111:69
Nieuwenhuys R (1963) The comparative anatomy of the actinopterygian forebrain. J Hirnforsch 6:171
Nieuwenhuys R (1964) Further studies on the general structure of the actinopterygian forebrain. Acta Morphol Neerl Scand 6:66–79
Nieuwenhuys R (1969) A survey of the structure of the forebrain in higher bony fishes (Osteichthyes). Ann NY Acad Sci 167:31
Nieuwenhuys R (1982) An overview of the organization of the brain of actinopterygian fishes. Am Zool 22:287–310
Nieuwenhuys R, Pouwels E (1983) The brain of actinopterygian fishes. In: Northcutt RG, Davis RE (eds) Fish neurobiology, vol 1. University of Michigan Press, Ann Arbor, pp 25–87
Northcutt RG, Braford MR Jr (1980) New observations on the organization and evolution of the telencephalon and actinopterygian fishes. In: Ebbesson SOE (ed) Comparative neurology of the telencephalon. Plenum Press, New York, pp 41–98
Ontoniente B, Tago H, Kimura H, Maeda T (1986) Distribution of GABA-inrmuoreactive neurons in the septal region of the rat brain. J Comp Neurol 248:422–430
Ottersen OP, Storm-Mathisen J (1984) Glutamate- and GABA-containing neurons in the mouse and rat brain as demonstrated with a new immunocytochemical technique. J Comp Neurol 229:374–392
Ottersen OP, Storm-Mathisen J (1985) Neurons containing or accumulating transmitter amino acids. In: Björklund A, Hökfelt T, Kulor MJ (eds) Handbook of chemical neuroanatomy, vol 3. Classical transmitters and transmitter receptors in the CNS. Part II. Elsevier, Amsterdam, pp 141–246
Parent A, Dubé L, Braford MR Jr, Northcutt RG (1978) The organization of monoamine-containing neurons in the brain of the sunfish (Lepomis gibbosus) as revealed by fluorescence microscopy. J Comp Neurol 182:495–516
Pasik P, Molinar-Rode R, Pasik T (1990) Chemically specified systems in the dorsal lateral geniculate nucleus of mammals. In: Cohen B, Bodis-Wollner I (eds) Vision and the brain. Raven Press, New York, pp 43–83
Peter REO, Kah O, Paulencu CR, Cook H, Kyle AL (1980) Brain lesions and short time endocrine effects of monosodium l-glutamate in goldfish, Carassius auratus. Cell Tissue Res 212:429–442
Rabhi M, Onteniente B, Kah O, Geffard M, Calas A (1987) Immunocytochemical study of the GABAergic innervation of the mouse pituitary by use of antibodies against GABA. Cell Tissue Res 247:33–40
Rio JP, Reperant J, Ward R, Micelli D, Medina M (1992) Evidence of GABA-immunoreactive neurons in the dorsal part of the lateral geniculate nucleus of reptiles: morphological correlates with interneurons. Neuroscience 47:395–407
Roberts BL, Meredith GE, Maslan S (1989) Immunocytochemical analysis of the dopamine system in the brain and spinal cord of the european eel, Anguilla anguilla. Anat Embryol 180:401–412
Roberts E, Chase TN, Tower DB (1976) GABA in nervous system function. Raven Press, New York
Rossi A, Capanna E, Tamino MG (1968) Prime osservazioni sulla citoarchitettonica cerebellare nei Teleostei. Accademia Nazionale dei Lincei, Rendiconti della classe di scienze fisiche, matematiche e naturali. Fasc. 5, Serie VIII, Vol XLV
Sas E, Maler L (1991) Retinofugal projections in a weakly electric gymnotid fish (Apteronotus leptorhynchus). Neuroscience 18:247–259
Sas E, Maler L, Tinner B (1990) Catecholaminergic systems in the brain of gymnotiform teleost fish: an immunohistochemical study. J Comp Neurol 292:127–162
Schimchowitsch S, Vuillez P, Tappaz ML, Klein MJ, Stoeckel ME (1991) Systematic presence of GABA-immunoreactivity in the tubero-infundibular and tubero-hypophyseal dopaminergic axonal system: an ultrastructural immunogold study on several mammals. Exp Brain Res 83:575–586
Schlussman SD, Kobylack MA, Dunn-Meynell AA, Sharma SC (1990) Afferent connections of the optic tectum in channel catfish Ictalurus punctatus. Cell Tissue Res 262:531–541
Schwerdtfeger WK, Lopez-Garcia C (1986) GABAergic neurons in the cerebral cortex of the brain of the lizard (Podarcis hispanica). Neurosci Lett 68:117–121
Schwerdtfeger W, Lorente MJ (1988) Laminar distribution and morphology of gamma-aminobutyric acid (GABA)-immunoreactive neurons in the medial and dorsomedial areas of the cerebral cortex of the lizard Podarcis hispanica. J Comp Neurol 278:473–485
Schwerdtfeger W, Lopez-Garcia C, Martinez-Guijarro FJ, Roberto PLT (1986) GABAergic neurons in the septum of the lizard, Podarcis hispanica. Brain Res 384:184–188
Seguela P, Geffard M, Buijs RM, Le Moal M (1984) Antibodies against gamma-aminobutyric acid: specificity studies and immunocytochemical results. Proc Natl Acad Sci USA 81:3888–3892
Seguela P, Gamrani H, Geffard M, Calas A, Le Moal M (1985) Ultrastructural immunocytochemistry of gamma-aminobutyrate in the cerebral and cerebellar cortex of the rat. Neuroscience 16:865–874
Sternberger LA (1979) Immunocytochemistry. Villeys, New York, p 345
Stoeckel ME, Tappaz M, Hindelang C, Seweryn C, Porte A (1985) Opposite effects of monosodium glutamate on the dopaminergic and GABAergic innervations of the median eminence and in the intermediate lobe in the mouse. Neurosci Lett 56:249–255
Striedter GF (1990a) The diencephalon of the channel catfish, Ictalurus punctatus. I. Nuclear organisation. Brain Behav Evol36:329–354
Striedter GF (1990b) The diencephalon of the channel catfish, Ictalurus punctatus. II. Retinal, tectal, cerebellar and telencephalic connections. Brain Behav Evol 36:355–377
Su YY, Wu JY, Lam DMK (1979) Purification of l-glutamic acid decarboxylase from catfish brain. J Neurochem 33:169–179
Sugden Ph, Newsholme EA (1977) Activities of cholineacetyltransferase, acetylcholinesterase, glutamate decarboxylase, 4-aminobutyrate aminotransferase in nervous tissue from some vertebrates and invertebrates. Comp Biochem Physiol 56C:89–94
Tappaz ML, Wassef M, Oertel VH, Paut L, Pujol JF (1983) Light and electron-microscopic immunocytochemistry of glutamic acid decarboxylase (GAD) in the basal hypothalamus: morphological evidence for neuroendocrine gamma-aminobutyrate (GABA). Neuroscience 9:271–283
Tumosa N, Stell WK (1986) Choline acetyltransferase immunoreactivity suggests that ganglion cells in the goldfish retina are not cholinergic. J Comp Neurol 244:265–270
Van der Heyden J, De Kloet ER, Korf J, Versteeg DHG (1979) GABA content of discrete brain nuclei and spinal cord of the rat. J Neurochem 33:857–861
Vincent SR, Hökelt T, Wu JY (1982) GABA neuron systems in the pituitary gland. Neuroendocrinology 34:117–125
Vuillez P, Crabajo Perez S, Stoeckel ME (1987) Colocalization of GABA and tyrosine hydroxylase immunoreactivities in the axons innervating the neurointermediate lobe of the rat pituitary: an ultrastructural immunogold study. Neurosci Lett 79:53–58
Watson AHD (1980) The distribution of aminergic neurons and their projections in the brain of the teleost, Myoxocephalus scorpius. Cell Tissue Res 208:299–312
Zandbergen MA, Peute J, Kallenbach AVH, Goos HJT (1991) The localization of GABA in the pituitary of the African catfish, Clarias gariepinus and the effect of GABA on the GTH release. In: Scott AP, Sumpter JP, Kime DE, Rolfe MS (eds) Proceedings of the Fourth International Symposium on the Reproductive Physiology of Fish. Norwich, Fish Symp 91, Sheffield, p 28
Zottoli SJ, Rhodes KJ, Mufson EJ (1987) Comparison of the acetylcholinesterase and choline acetyltransferase staining patterns in the optic tectum of the goldfish Carassius auratus. A histochemical and immunocytochemical analysis. Brain Behav Evol 30:143–159
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Médina, M., Repérant, J., Dufour, S. et al. The distribution of GABA-immunoreactive neurons in the brain of the silver eel (Anguilla anguilla L.). Anat Embryol 189, 25–39 (1994). https://doi.org/10.1007/BF00193127
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00193127