Summary
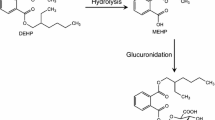
In vitro experiments were performed with cytosolic and microsomal fractions of human liver specimens in order to investigate which enzyme forms of sulfotransferase (ST) and UDP-glucurosyltransferase (GT) are involved in the metabolism of digitoxin (dt-3) and/or its cleavage products. It was found that the cytosolic STs preferentially react with digitoxigenin (dt-0) whereas microsomal GTs conjugate digitoxigenin-monodigitoxoside (dt-1) and in traces the bisdigitoxoside (dt-2). Dt-3 and dt-0 cannot be glucuronidated. By separation of different sulfotransferases it was found that the hydroxysteroid-ST is responsible for dt-0 and 3-epidigitoxigenin (epi-dt-0) sulfation. The hydroxysteroid-ST could be purified and characterized (apparent Km and Vmax for dt-0 sulfation: approx. 17 μmol/l and 2.7 nmol/min mg protein, respectively). Of various model substrates and endogenous compounds (steroids, bilirubin) none caused a competitive inhibition of the microsomal dt-1 glucuronidation except dt-2 and dt-3. Therefore it can be supposed that a new GT form catalyses this reaction. It is characterized by an extraordinarily high affinity towards dt-1 with Km values ranging between 0.7 and 27 μmol/l.
Similar content being viewed by others
Abbreviations
- DHEA:
-
dehydroepiandrosterone
- dg-0:
-
digoxigenin
- dg-1:
-
digoxigenin-monodigitoxoside
- dt-0:
-
digitoxigenin
- dt1:
-
digitoxigenin-monodigitoxoside
- dt-2:
-
digitoxigenin-bisdigitoxoside
- dt-3:
-
digitoxigenin-trisdigitoxoside, digitoxin
- epi-dt-0:
-
3-epi-digitoxigenin
- GT:
-
UDP-glucuronosyltransferase
- PAPS:
-
3′-phosphoadenosine-5′-phosphosulfate
- ST:
-
sulfotransferase
- UDPGA:
-
UDP-glucuronic acid
References
Bock KW, Josting D, Lilienblum W, Pfeil H (1979) Purification of rat liver microsomal UDP-glucurosyltransferase. Separation of two enzyme forms inducible by 3-methylcholanthrene or phenobarbital. Eur J Biochem 98:19–26
Campbell NR, Van Loon JA, Weinshilboum RM (1987) Human liver phenol sulfotransferase: Assay conditions, biochemical properties and partial purification of isoenzymes of the thermostable form. Biochem Pharmacol 36:1435–1446
Castle MC (1980) Glucuronidation of digitalis glycosides by rat liver microsomes. Stimulation by spironolactone and pregnenolone 16α-carbonitrile. Biochem Pharmacol 29:1497–1502
Castle MC, Lage GL (1974) Cleavage by β-glucuronidase of the water-soluble metabolites of digitoxin excreted in the bile of control and spironolactone-pretreated rats. Toxicol Appl Pharmacol 27:641–647
Falany CN, Vazquez ME, Kalb JM (1989) Purification and characterization of human liver dehydroepiandrosterone sulphotransferase. Biochem J 260:641–646
Falany CN, Vazquez ME, Heroux JA, Roth JA (1990) Purification and characterization of human liver phenol-sulfating phenol sulfotransferase. Arch Biochem Biophys 278:312–318
Gault MH, Charles JD, Sudgen DL, Kepkay DC (1977) Hydrolysis of digoxin by acid. J Pharm Pharmacol 29:27–32
Herrmann I, Repke K (1963) Über das Glucuronsäurekonjugat eines Cardenolidgenins (3-Epi-digitoxigenin). Experientia XIX/9:472–474
Hey G, Von der Eldern-Delbrügge U (1982) Cytochrome P-450 dependent metabolism of digitoxigenin digitoxosides in different species. Naunyn-Schmiedeberg's Arch Pharmacol 319 [Suppl]: 170
Irshaid YM, Tephly TR (1987) Isolation and purification of two human liver UDP-glucuronosyltransferases. Mol Pharmacol 31:27–34
Johannes A, Von Meyerinck L, Schmoldt A (1990) Purification of a rat liver cytosolic sulfotransferase responsible for the conjugation of digitoxigenin. Biochem Pharmacol 39:301–307
Kutt H, Fouts JR (1971) Diphenylhydantoin metabolism by rat liver microsomes and some effects of drug or chemical pretreatment on the diphenylhydantoin metabolism by rat liver microsomal preparations. J Pharmacol Exp Ther 176:11–18
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 227:680–685
Miners JO, Lillywhite KJ, Birkett DJ (1988) In vitro evidence for the involvement of at least two forms of human liver UDP-glucuronosyltransferase in morphine 3-glucuronidation. Biochem Pharmacol 37:2839–2845
Powis G, Jardine I, Van Dyke R, Weinshilboum R, Moore D, Wilke D, Rhodes W, Nelson R, Benson L, Szulanski C (1988) Foreign compound metabolism studies with human liver obtained as surgical waste. Relation to donor characteristics and effect of tissue storage. Drug Metab Dispos 16:582–589
Radominska A, Comer KA, Zimniak P, Falany J, Iscan M, Falany CN (1990) Human liver steroid sulfotransferase sulphates bile acids. Biochem J 272:597–604
Rein G, Glover V, Sandler M (1982) Multiple forms of phenolsulfotransferases in human tissues. Selective inhibition by dichloronitrophenol. Biochem Pharmacol 31:1893–1897
Reiter C, Mwaluko G, Dunnette J, Van Loon J, Weinshilboum R (1983) Thermolabile and thermostable human platelet phenol sulfotransferase: substrate specificities and physical separation. Naunyn-Schmiedeberg's Arch Pharmacol 324:140–147
Satoh D, Aoyama K (1970) Studies on digitalis glycosides. XXXI. Stepwise degradation of polydigitoxosides of cardenolides: Chem Pharm Bull 18:94–99
Schmoldt A (1978) Increased digitoxin cleavage by liver microsomes of spironolactone-pretreated rats. Naunyn-Schmiedeberg's Arch Pharmacol 305:261–263
Schmoldt A (1984) Untersuchungen zum Metabolismus der Digitalisglycoside. Beitr Gerichtl Med 17:95–101
Schmoldt A, Von Meyerinck L, Drohn W, B1ömer I (1986) Enzymatic basis for digitoxin metabolism and possible drug interactions in man. In: Erdman E, Greeff K, Skou JC (eds) Cardiac glycosides. Springer, New York Berlin Heidelberg, pp 273–279
Schmoldt A, Herzfeldt B, VonMeyerinck L, Benthe HF (1987) Evidence for a digitoxin conjugating UDP-glucuronosyltransferase in the dog. Biochem Pharmacol 36:3951–3955
Schmoldt A, Promies J (1982) On the substrate specificity of the digitoxigenin monodigitoxoside conjugating UDP-glucuronosyltransferase in rat liver. Biochem Pharmacol 31:2285–2289
Schmoldt A, Rohloff C (1978) Dehydro-digitoxosides of digitoxigenin: Formation and importance for the digitoxin metabolism in the rat. Naunyn-Schmiedeberg's Arch Pharmacol 305:167–172
Tephly TR, Townsend M, Green MD (1989) UDP-glucuronosyltransferases in the metabolic disposition of xenobiotics. Drug Metab Dispos 20:689–695
Tukey RH, Tephly TR (1981) Purification and properties of rabbit liver estrone and p-nitrophenol UDP-glucuronosyltransferase. Arch Biochem Biophys 209:565–578
Vöhringer HF, Rietbrock N (1974) Metabolism and excretion of digitoxin in man. Clin Pharmacol 16:796–806
Vöhringer HF, Rietbrock N (1978) Pharmacokinetics and metabolism of digitoxin in the human. In: Bodem G, Dengler HJ (eds) International Symposium on Cardiac Glycosides, Bonn 1977. Springer, Berlin Heidelberg New York, pp 64–71
Von Meyerinck L, Coffman BL, Green MD, Kirkpatrick RB, Schmoldt A, Tephly TR (1985) Separation, purification, and characterization of digitoxigenin-monodigitoxide UDP-glucuronosyltransferase activity. Drug Metab Dispos 13: 700–704
Zilly W (1979) Digitoxin bei akuter und chronischer Leberinsuffizienz. In: Greeff K, Rietbrock N (eds) Digitoxin als Alternative in der Therapie der Herzinsuffizienz. Schattauer, Stuttgart New York, pp 199–212
Author information
Authors and Affiliations
Additional information
Send offprint requests to A. Schmoldt at the above address
Rights and permissions
About this article
Cite this article
Schmoldt, A., Blömer, I. & Johannes, A. Hydroxysteroid sulfotransferase and a specific UDP-glucuronosyltransferase are involved in the metabolism of digitoxin in man. Naunyn-Schmiedeberg's Arch Pharmacol 346, 226–233 (1992). https://doi.org/10.1007/BF00165306
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00165306