Abstract
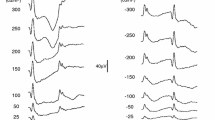
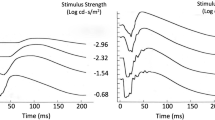
The corneally recorded rod photocurrent component (photoresponse) underlying the a-wave feature of the electroretinogram was analyzed. The results set empiric limits on critical photoresponse variables. Measurements were obtained from four normal adult subjects on a-wave amplitude, a-wave velocity, b-wave amplitude, b-wave implicit time and b-wave height above baseline. At high intensity, interference from the b-wave component was minimized and the amplitude of the saturated photoresponse component was approximated by the a-wave feature. At lower intensities, the a-wave feature represented progressively less of the underlying photoresponse amplitude. Photoresponse amplitude saturation was signaled by the abrupt slowing of the rate of decline of b-wave peak latency and occurred at an intensity about 2.5 log units above the first appearance of the b-wave. At the intensity of photoresponse saturation, the peak amplitude of the a-wave feature was only about 25% of the maximum amplitude of the underlying photoresponse component. A-wave leading edge velocity was found to increase up to 3 log units above the intensity of photoresponse amplitude saturation and to provide a good estimate of photoresponse velocity at higher intensities. A cascaded low-pass filter model with modifications to accommodate amplitude and timing nonlinearities was used to generate a set of probable underlying photoresponses from the analysis of a-wave amplitude and velocity. Movement of the a-wave leading edge to the left at higher intensities in algebraic combination with a static b-wave leading edge above the intensity of photoresponse amplitude saturation was found to explain the second rise of the b-wave amplitude function and the decline of b-wave amplitude above baseline at high intensities. This analysis provides a basis for modeling the underlying photoresponse on a biochemical level and for interpreting photoreceptor damage in disease states.
Similar content being viewed by others
References
Johnson MA, Marcus S, Elman MJ, McPhee TJ. Electroretinographic abnormalities associated with neovascularization in central retinal vein occlusion. Arch Ophthalmol 1988; 106: 348–52.
Kaye SB, Harding SP. Early electroretinography in unilateral central retinal vein occlusion as a predictor of rubeosis iridis. Arch Ophthalmol 1988; 106: 353–6.
Breton ME, Quinn GE, Keene SS, et al. Electroretinogram parameters at presentation as predictors of rubeosis in central retinal vein occlusion patients. Ophthalmology 1989; 96: 1343–52.
Bresnich GH, Palta M. Temporal aspects of the electroretinogram in diabetic retinopathy. Arch Ophthalmol 1987; 105: 660–4.
Breton ME, Montzka DP, Brucker AJ, Quinn GE. Electroretinogram interpretation in central retinal vein occlusion. Ophthalmology. 1991; 98: 1837–44.
Granit R. The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J Physiol 1933; 77: 207–39.
Arden GB. The retina. Neurophysiology. In: The eye. New York: Academic Press, 1976; chap. 7.
Penn RD, Hagins WA. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature 1969; 223: 201–5.
Stryer L. Molecular basis of visual excitation. Cold Spring Harbor Symposia on Quantitative Biology 1988; 53.
Pugh EN, Lamb TD. Minireview. Cyclic GMP and calcium. The internal messengers of excitation and adaptation in vertebrate photoreceptors. Vision Res 1990; 30: 1923–48.
Hagins WA, Penn RD, Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J 1970; 10: 380–412.
Penn RD, Hagins WA. Kinetics of the photocurrent of retinal rods. Biophys J 1972; 12: 1073–94.
Baylor DA, Lamb TB, Yau KW. The membrane current of single rod outer segments. J Physiol 1979; 288: 589–611.
Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey macaca fascicularis. J Physiol 1984; 357: 575–607.
Hood DC, Birch DG. The a-wave of the human electroretinogram and rod receptor function. Invest Ophthalmol Vis Sci 1990; 31: 2070–81.
Hood DC, Birch DG. A computational model of the amplitude and implicit time of the b-wave of the human ERG. Vis Neurosci. 1992; 8: 107–26.
Peachey NS, Alexander KR, Fishman GA. The luminance-response function of the dark-adapted human electroretinogram. Vision Res 1989; 29: 263–70.
Sieving PA, Nino C. Scotopic threshold response (STR) of the human electroretinogram. Invest Ophthalmol Vis Sci 1988; 11: 1608–14.
Brown KT, Murakami M. A new receptor potential of the monkey retina with no detectable latency. Nature 1964; 201: 626–8.
Brindley GS, Gardner-Medwin AR. The origin of the early receptor potential of the retina. J Physiol 1966; 182: 185–94.
Birch DG, Fish GE. Rod ERGs in retinitis pigmentosa and cone-rod degeneration. Invest Ophthalmol Vis Sci 1987; 28: 140–50.
Wyszecki G, Stiles WS. Color science. Concepts and methods, quantitative data and formulas. New York: John Wiley & Sons, 1982: 104.
Pugh EN Jr. Vision. Physics and retinal physiology. In: Atkinson RC, Herrnstein RJ, Lindzey G, Luce RD, eds. Stevens' handbook of experimental psychology. New York: John Wiley & Sons, 1988: 75–87.
Cobbs WH, Pugh EN Jr. Kinetics and components of the flash photocurrent of isolated retinal rods of the larval salamander, Ambystoma tigrinum. J Physiol 1987; 394: 529–72.
Newman EA, Odette LL. Model of electroretinogram b-wave generation. A test of the K+ hypothesis. Neurophysiol 1984; 51: 164–82.
Rodieck RW. The vertebrate retina. Principles of structure and function. San Francisco: W. H. Freeman and Co., 1973: 526–58.
Johnson MA, Massof RW. The photomyoclonic reflex. An artifact in the clinical electroretinogram. Br Ophthalmol 1988; 66: 368–78.
Montzka DP, Breton ME, Schueller AW. Cone intrusion in white and blue flash ERG a-wave velocity measurements [Abstract]. Invest Ophthalmol Vis Sci 1991; 32: 928.
Hood DC, Birch DG. A quantitative measure of the electrical activity of human rod photoreceptors using electroretinography. Vis Neurosci 1990; 5: 379–87.
Author information
Authors and Affiliations
Additional information
Supported in part by a grant from the Ethel Brown Foerderer Foundation, Children's Hospital of Philadelphia, and by a grant from the Nina and Paul MacKall Trust.
Rights and permissions
About this article
Cite this article
Breton, M.E., Montzka, D.P. Empiric limits of rod photocurrent component underlying a-wave response in the electroretinogram. Doc Ophthalmol 79, 337–361 (1992). https://doi.org/10.1007/BF00160948
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00160948