Summary
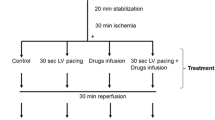
31-P NMR spectroscopy data recorded for the isolated heart were analyzed, in conjunction with functional and biochemical variables, in order to investigate the effect observed for several different beta-adrenoceptor antagonists on the alterations provoked by global partial ischemia (37°C, 24 minutes, 1% residual coronary flow) and reperfusion in the metabolism of the myocardium. During ischemia: intracellular acidosis, adenosine triphosphate (ATP) degradation, and inorganic phosphate (Pi) accumulation were found to be reduced whether the perfusion fluid contained: acebutolol 2.7×10-5 M, atenolol 10-5 M, d-propranolol 10-5 M, or dl-propranolol 10-5 M. On reperfusion metabolic and functional variables were variously affected by the different drugs, except the Pi level which was, in all series, significantly lower compared with control hearts. The adenylate charge and the glycogen stores were protected in the acebutolol, dl-propranolol, and d-propranolol groups. The ATP level was higher than in controls only in the acebutolol and atenolol groups. The intracellular pH recovered to values non-significantly different from preischemic values in the acebutolol and dl-propranol-treated hearts only. The mechanical performance, expressed as the rate-pressure product, was unaltered by the ischemia-reperfusion sequence in the acebutolol and d-propranolol series, while decreasing significantly in controls and in the atenolol group. In dl-propranolol-treated hearts the mechanical activity, which in normoxic conditions was already halved during the effect of the drug, remained at this same level after ischemia. From these observations, it appears that the nonspecific properties of the drugs, as distinct from beta-blockade, play an important part in attenuating the ischemia-induced alteration in myocardial metabolism. Thus, it can be postulated that (1) the metabolic effects of dl-propranolol probably result largely from the reduction of heart work induced by this drug; (2) the maintenance of energy metabolism associated with the preservation of the myocardial activity, as observed in the case of acebutolol and d-propranolol, is possibly a consequence of the existence of a membrane-stabilizing activity.
Similar content being viewed by others
References
Bush LR, Haack DW, Schlafer M, et al. Protective effects of beta-adrenergic blockade in isolated ischemic hearts. Eur J Pharmacol 1980;67:209–217.
De Leiris J, Peyrot M, Feuvray D. Pharmacological reduction of ischemia-induced enzyme release from isolated rat heart. J Mol Med 1978;3, 111–121.
Rochette L, Didier JP, Moreau D, et al. Role of beta-adrenoceptor antagonism in the prevention of reperfusion ventricular arrhythmias: Effects of acebutolol, atenolol and D-propranolol on isolated working rat hearts subject to myocardial ischemia and reperfusion. Am Heart J 1987; 107:1132–1141.
Daugherty A, Frayn KN, Redfeen WS, et al. The role of catecholamines in the production of ischaemia-induced ventricular arrhythmias in the rat in vivo and in vitro. Br J Pharmacol 1966;87:265–277.
Lavanchy N, Martin J, Rossi A. Graded global ischaemia and reperfusion of the isolated rat heart: Characterization by 31-P NMR spectroscopy of the extent of energy metbolism damage. Cardiovasc Res 1984;18:573–582.
Pieper GM, Todd ST, Wu JM, et al. Attenuation of myocardial acidosis by propranolol during ischaemic arrest and reperfusion: Evidence with 31P nuclear magnetic resonance. Cardiovasc Res 1980;14:646–653.
Nakasawa M, Katano Y, Imai S, et al. Effects of 1-and d-propranolol on the ischemic myocardial metabolism of the isolated guinea pig heart, as studied by 31P NMR. J Cardiovasc Pharmacol 1982;4:700–704.
Parmley WW, Braunwald E. Comparative myocardial depressant and anti-arrhythmic properties of d-propranolol, dl-propranolol and quinidine. J Pharmacol Exp Ther 1967; 158:11–21.
Nayler WG, Stone J, Carson V, et al. The effect of bata-adrenergic antagonists on cardiac contractions, myofibrillar ATPase activity, high-energy phosphate stores and lipid facilitated transport of calcium ions. J Pharmacol Exp Ther 1969;165:225–233.
Lavanchy N, Martin J, Giacotielli M, et al. Evaluation by 31-P NMR of the effects of acebutolol on the ischaemic isolated rat heart. Eur J Pharmacol 1986;125:341–351.
Barett AM, Cullum VA. The biological properties of the optical isomers of propranolol and their effects on cardiac arrhythmias. Br J Pharmacol 1968;34:43–55.
Frishman W, Clinical pharmacology of the new beta-adrenergic blocking drugs. Part 1. Pharmacodynamic and pharmacokinetic properties. Am Heart J 1979;97:663–670.
Lavanchy N, Martin J, Rossi A. The role of beta-adrenoceptors in ischemia-induced acidosis in the isolated rat heart: A 31-P NMR study. In: Dhalla NS, Innes IR, Beamish RE, eds. Myocardial Ischemia. Boston: Martinus Nijhoff, 1987;199–212.
Coltart DH, Gibson DG, Shand DG. Plasma propranolol levels associated with suppression of ventricular ectopic beats. Br Med J 1971;1:490–491.
Woosley RL, Kornhauser D, Smith R, et al. Suppression of chronic ventricular arrhythmias with propranolol. Circulation 1979;60:819–827.
Davis WG, Tente G. Effects of propranolol on the transmembrane potentials of ventricular muscle and Purkinje fibers of the dog. Circ Res 1968;22:661–667.
Arnim TV, Welman E. Propranolol in ischaemic-reperfused working rat heart: Dissociation of beta-adrenergic blocking and protective effect. J Mol Cell Cardiol 1981;13:521–524.
Manning AS, Keogh JM, Coltart DJ, et al. Propranolol in the ischaemic, reperfused, working rat heart: Association between beta-adrenergic blocking activity and protective effect. J Mol Cell Cardiol 1981;13:1077–1080.
Dhalla NS, Lee SL, Anand MB, et al. Effects of acebutolol, practolol and propranolol on the rat heart sarcolemma. Biochem Pharmacol 1977;26:2055–2060.
Bhayana V, Alto LE, Dhalla NS. The effects of β-adernergic receptor blockers on heart mitochondrial metabolism. Gen Pharmacol 1980;11:271–274.
Brink AT, Bester AJ, Lochner A. Effect of propranolol on the metabolism and function of the rat heart. J Mol Cell Cardiol 1971;2:71–89.
Wei YH, Lin TN, Hong CY, et al. Inhibition of the mitochondrial Mg2+ — ATPase by propranolol. Biochem Pharmacol 1985;34:911–917.
Sobel B, Jequier E, Sjoerdsma A, et al. Effects of catecholamines and adrenergic blocking agents on oxidative phosphorylation in rat heart mitochondria. Circ Res 1966; 19:1050–1060.
Smith HJ. The need to redefine membranes stabilizing activity of beta-adrenergic receptor antagonists. J Mol Cell Cardiol 1982;14:495–500.
Davis WG. A comparison of the local anaesthetic “quinidine-like” and adrenergic β-blocking-activities of fibe β-receptor antagonists. J Pharm Pharmacol 1970;22:284–290.
Wollenberger A, Shahab L. Anoxia-induced release of noradrenaline from the isolated perfused heart. Nature 1965;207:88–89.
Lammerant J, Dehert P, Deschryver C. Direct release of catecholamines into the left heart chambers: The enhancing effect of acute coronary occlusion. Arch Int Pharmacodyn 1966;163:219–226.
McGrath BP, Lim SP, Leversha L, et al. Myocardial and peripheral catecholamine response to acute coronary artery constriction before and after propranolol treatment in the anaesthetized dog. Cardiovasc Res 1981;15:28–34.
Riemersma RA, Forfar JC. Myocardial noradrenaline release during acute coronary occlusion. Eur J Clin Invest 1981; 11:26–38.
Rochette L, Didier JP, Moreau D, et al. Effects of substrate on release of myocardial epinephrine and ventricular arrhythmias following reperfusion of the ischemic isolated working rat heart. J Cardiovasc Pharmacol 1980;2:267–279.
Abrahamsson T, Almgren O, Carlsson L. Ischemia-induced noradrenaline release in the isolated rat heart: Influence of perfusion substrate and duration of ischemia. J Mol Cell Cardiol 1983;15:821–830.
Carlsson L, Abrahamsson T, Almgren O. Release of noradrenaline in myocardial ischemia. Importance of local inactivation by neuronal and extraneuronal mechanisms. J Cardiovasc Pharmacol 1986;8:545–553.
Kissin I, Cavender JB. Effect of propranolol on energy demand-supply balance in myocardial ischemia. J Cardiovasc Pharmacol 1981;3:992–1001.
Lubbe WF, Muller CA, Worthington M, et al. Influence of propranolol isomers and or atenolol on myocardial cyclic AMP, high energy phosphates and vulnerability to fibrillation after coronary artery ligation in the isolated rat heart. Cardiovasc Res 1981;15:690–699.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lavanchy, N., Martin, J. & Rossi, A. Comparative study of the effects of acebutolol, atenolol, d-propranolol and dl,-propranolol on the alterations in energy metabolism caused by ischemia and reperfusion: A 31P NMR study on the isolated rat heart. Cardiovasc Drug Ther 2, 501–512 (1988). https://doi.org/10.1007/BF00051189
Issue Date:
DOI: https://doi.org/10.1007/BF00051189