Abstract
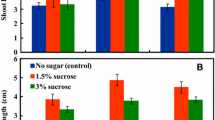
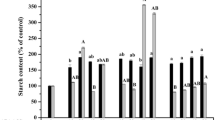
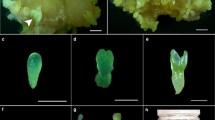
The effects of sucrose on maturation and of plant growth regulators on germination of soybean somatic embryos were investigated for the purpose of developing an efficient culture method for plant recovery. Somatic embryos produced on medium with a low sucrose concentration (5 gl-1), less than 1 mm in length, 0.6 mg in fresh weight, and green in color, were grown for 2 weeks on MS medium containing 5 gl-1 or 30 gl-1 sucrose and then for another 5 weeks on MS medium containing 5–90 gl-1 sucrose. The highest increase in fresh weight of somatic embryos was obtained in the treatment of transferring from 30 gl-1 sucrose (2 weeks) to 60 gl-1 (5 weeks). With the increase in fresh weight, the somatic embryos gradually changed color from green to yellow, and finally to white, when they stopped growth. Soybean seed storage proteins (β-conglycinin and glycinin) were accumulated in somatic embryos under tissue specific and stage specific control analogous to that in zygotic embryos. Exogenous gibberellic acid was effective in promoting precocious germination of premature soybean somatic embryos, but was not necessary for the germination of mature somatic embryos. The efficiency of somatic embryo germination was as high as 77% from semi-wild soybean and 60–64% from cultivated soybeans, showing that the plant regeneration system developed in this study was efficient and practical.
Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- BR:
-
brassinolide
- GA3 :
-
gibberellic acid
- IBA:
-
indolebutyric acid
- NAA:
-
α-naphthaleneacetic acid
- PAGE:
-
polyacrylamide gel electrophoresis
- SDS:
-
Sodium Lauryl Sulfate
References
Allavena A (1984) Beans (Phaseolus) In: Sharp WR, Evans DA, Ammirato PV & Yamada Y (Eds) Handbook of Plant Cell Culture, Vol 2, Crop Species (pp 137–168). Macmillan, New York
Ammirato PV (1989) Recent progress in somatic embryogenesis. IAPTC Newsletter 57: 2–16
Bajaj YPS (1984) Peanut In: Ammirato PV, Evans DA, Sharp WR & Yamada Y (Eds) Handbook of Plant Cell Culture, Vol 3, Crop Species (pp 193–225). Macmillan, New York
Barwale UB & Widholm JM (1987) Somaclonal variation in plants regenerated from cultures of soybean. Plant Cell Rep. 6: 365–368
Barwale UB, Kerns HR & Widholm JM (1986) Plant regeneration from callus cultures of several soybean genotypes via embryogenesis and organogenesis. Planta 167: 473–481
Buchheim JA, Colburn SM & Ranch JP (1989) Maturation of soybean somatic embryos and the transition to plant growth. Plant Physiol. 89: 768–775
Christianson ML, Warnick DA & Carlson PS (1983) A morphogenetically competent soybean suspension culture. Science 222: 632–634
Crouch ML (1982) Non-zygotic embryos of Brassica napus L. contain embryo-specific storage proteins. Planta 156: 520–524
Crouch ML & Sussex IM (1981) Development and storageprotein synthesis in Brassica napus L. embryos in vivo and in vitro. Planta 153: 64–74
Gamborg OL, Miller RA & Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151–158
Kageyama C, Komatsuda T & Nakajima K (1990) Effects of sucrose concentration on morphology of somatic embryos from immature soybean cotyledons. Plant Tissue Culture Lett. 7: 108–110
Khan AA (1982) Gibberellins and seed development. In: Khan AA (Ed) The Physiology and Biochemistry of Seed Development, Dormancy and Germination (pp 111–135). Elsevier Biomedical, Amsterdam
Komatsuda T (1990) Ability of soybean (Glycine max L. Merr.) genotypes to produce somatic embryos on a medium containing a low concentration of sucrose. Japan J. Breed. 40: 371–375
Komatsuda T & Ohyama K (1987) Screening of genotypes for somatic embryogenesis and plant regeneration in soybean. Japan J. Breeding 37 (suppl 1): 54–55 (in Japanese with English tables and figures)
Komatsuda T & Ohyama K (1988) Genotypes of high competence for somatic embryogenesis and plant regeneration in soybean Glycine max. Theor. Appl. Genet. 75: 695–700
Komatsuda T, Kang S & Oka S (1990a) A somaclonal mutant of soybean seed protein. Abstracts VIIth International Congress on Plant Tissue and Cell Culture (A4-57). Amsterdam
Komatsuda T, Oka S, Miyasaka Y & Zhang XZ (1990b) Plant regeneration from protoplasts via embryogenesis in soybean. In: Shoemaker R (Ed) Proc 3rd Biennial Conference on Molecular and Cellular Biology of the Soybean (p 5). 23–25 July 1990, Iowa State University, Iowa
Komatsuda T, Kaneko K & Oka S (1991) Genotype × sucrose interactions for somatic embryogenesis in soybean. Crop. Sci. 31: 333–337
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Lazzeri PA, Hildebrand DF & Collins GB (1985) A procedure for plant regeneration from immature cotyledon tissue of soybean. Plant Mol. Biol. Rep. 3: 160–167
Lazzeri PA, Hildebrand DF & Collins GB (1987a) Soybean somatic embryogenesis: Effects of hormones and culture manipulations. Plant Cell Tiss. Org. Cult. 10: 197–208.
Lazzeri PA, Hildebrand DF & Collins GB (1987b) Soybean somatic embryogenesis: Effects of nutritional, physical and chemical factors. Plant Cell Tiss. Org. Cult. 10: 209–220
Lazzeri PA, Hildebrand DF, Sunega J, Williams EG & Collins GB (1988) Soybean somatic embryogenesis: interactions between sucrose and auxin. Plant Cell Rep. 7: 517–520
Lippmann B & Lippman G (1984) Induction of somatic embryos in cotyledonary tissue of soybean, Glycine max L. Merr. Plant Cell Rep. 3: 215–218
McCoy T & Walker K (1984) Alfalfa. In: Ammirato PV, Evans DA, Sharp WR & Yamada Y (Eds) Handbook of Plant Cell Culture, Vol 3. Crop Species (pp 171–192). Macmillan, New York
Meinke DW, Chen J & Beachy RN (1981) Expression of storage-protein genes during soybean seed development. Planta 153: 130–139
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Parrott WA, Hoffman LM, Hildebrand DF, Williams EG & Collins GB (1989) Recovery of primary transformants of soybean. Plant Cell Rep. 7: 615–617
Phillips GC & Collins GB (1984) Red clover and other forage legumes In: Sharp WR, Evans DA, Ammirato PV & Yamada Y (Eds) Handbook of Plant Cell Culture, Vol 2. Crop Species (pp 169–210). Macmillan, New York
Ranch JP, Oglesby L & Zielinski AC (1985) Plant regeneration from embryo-derived tissue cultures of soybeans. In Vitro Cellular & Developmental Biol. 21: 653–658
Reinert J (1973) Aspects of organization-organogenesis and embryogenesis. In: Street HE (Ed) Plant Tissue and Cell Culture (pp 338–355). Blackwell, Oxford/London/Edinburgh/Melbourne
Shoemaker RC, Christofferson SE & Galbraith DW (1987) Storage protein accumulation patterns in somatic embryos of cotton (Gossypium hirsutum L). Plant Cell Rep. 6: 12–15
Tilton VR & Russell SH (1984) In vitro culture of immature soybean embryos. J. Plant Physiol. 115: 191–200
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Komatsuda, T., Lee, W. & Oka, S. Maturation and germination of somatic embryos as affected by sucrose and plant growth regulators in soybeans Glycine gracilis Skvortz and Glycine max (L.) Merr.. Plant Cell Tiss Organ Cult 28, 103–113 (1992). https://doi.org/10.1007/BF00039922
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00039922