Abstract
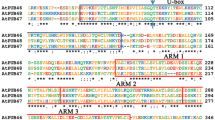
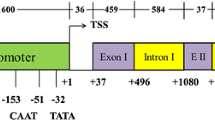
Ubiquitin is an omnipresent protein found in all eukaryotes so far analysed. It is involved in several important processes, including protein turnover, chromosome structure and stress response. Parsley (Petroselinum crispum) contains at least two active polyubiquitin (ubi4) genes encoding hexameric precursor proteins. The deduced amino acid sequences of the ubiquitin monomers are identical to one another and to ubiquitin sequences from several other plant species. Analysis of the promoter region of one ubi4 gene revealed putative regulatory elements. In parsley plants, the ubi4 mRNAs were the predominant ubiquitin mRNAs and were present at comparable levels in all plant organs tested. In cultured parsley cells, high levels of ubiquitin gene expression remained unaffected by heat shock, elicitor or light treatment.
Similar content being viewed by others
References
Armstrong GA, Weisshaar B, Hahlbrock K: Homodimeric and heterodimeric leucine zipper proteins and nuclear factors from parsley recognize diverse promoter elements with ACGT cores. Plant Cell 4: 525–537 (1992).
Bachmair A, Becker F, Masterson RV, Schell J: Perturbation of the ubiquitin system causes leaf curling, vascular tissue alterations and necrotic lesions in a higher plant. EMBO J 9: 4543–4549 (1990).
Baker RT, Board PG: The human ubiquitin gene family: structure of a gene and pseudogenes from the Ub B subfamily. Nucl Acids Res 15: 443–463 (1987).
Binet M-N, Lepetit M, Weil J-H, Tessier L-H: Analysis of a sunflower polyubiquitin promoter by transient expression. Plant Sci 79: 87–94 (1991).
Binet M-N, Steinmetz A, Tessier L-H: The primary structure of sunflower (Helianthus annuus) ubiquitin. Nucl Acids Res 17: 2119 (1989).
Binet M-N, Weil JH, Tessier LH: Structure and expression of sunflower ubiquitin genes. Plant Mol Biol 17: 395–407 (1991).
Bond U, Schlesinger MJ: The chicken ubiquitin gene contains a heat shock promoter and expresses an unstable mRNA in heat-shocked cells. MCB 6: 4602–4610 (1986).
Bouchez D, Tokuhisa JG, Llewellyn DJ, Dennis ES, Ellis JG: The ocs-element is a component of the promoters of several T-DNA and plant viral genes. EMBO J 8: 4197–4204 (1989).
Brown JWS: A catalogue of splice junction and putative branch point sequences from plant introns. Nucl Acids Res 14: 9549–9559 (1986).
Burke TJ, Callis J, Vierstra RD: Characterization of a polyubiquitin gene from Arabidopsis thaliana. Mol Gen Genet 213: 435–443 (1988).
Callis J, Raasch JA, Vierstra RD: Ubiquitin extension proteins of Arabidopsis thaliana. J Biol Chem 265: 12486–12493 (1990).
Christensen AH, Quail PH: Sequence analysis and transcriptional regulation by heat shock of polyubiquitin transcripts from maize. Plant Mol Biol 12: 619–632 (1989).
Christensen AH, Sharrock RA, Quail PH: Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18: 675–689 (1992).
Devereux J, Haeberli P, Smithies O: A comprehensive set of sequence analysis programs for the VAX. Nucl Acids Res 12: 387–395 (1984).
Douglas C, Hoffmann H, Schulz W, Hahlbrock K: Structure and elicitor or U.V.-light-stimulated expression of two 4-coumarate:CoA ligase genes in parsley. EMBO J 6: 1189–1195 (1987).
Efstratiadis A, Posakony JW, Manatis T, Lawn RM, O'Connel C, Spritz RA, DeRiel JK, Forget BG, Weissman SM, Slightom JL, Blechl AE, Smithies O, Baralle FE, Shoulders CC, Proodfoot NJ: The structure and evolution of the human ß-globin gene family. Cell 21: 653–668 (1980).
Feinberg AP, Vogelstein B: A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6–13 (1983).
Finley D, Bartel B, Varshavsky A: The tails of ubiquitin precursors are ribosomal proteins whose fusion to ubiquitin facilitates ribosome biogenesis. Nature 338: 394–401 (1989).
Finley D, Chau V: Ubiquitination. Annu Rev Cell Biol 7: 25–69 (1991).
Frohman MA, Dush MK, Martin GR: Rapid production of full-length cDNAs from rare transcripts: Amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci USA 85: 8998–9002 (1988).
Gausing K, Barkardottir R: Structure and expression of ubiquitin genes in higher plants. Eur J Biochem 158: 57–62 (1986).
Giuliano G, Pichersky E, Malik VS, Timko MP, Scolnic PA, Cashmore AR: An evolutionary conserved protein binding sequence upstream of a plant light-regulated gene. Proc Natl Acad Sci USA 85: 7089–7093 (1988).
Gurley WB, Key JL: Transcriptional regulation of the heat-shock response: A plant perspective. Biochemistry 30: 1–12 (1991).
Herrmann A, Schulz W, Hahlbrock K: Two alleles of the single-copy chalcone synthase gene in parsley differ by a transposon-like element. Mol Gen Genet 212: 93–98 (1988).
Hoffman NE, Ko K, Milkowski D, Pichersky E: Isolation and characterization of tomato cDNA and genomic clones encoding the ubiquitin gene ubi3. Plant Mol Biol 17: 1189–1201 (1991).
Jabben M, Shanklin J, Vierstra RD: Ubiquitin-phytochrome conjugates: Pool dynamics during in vitro phytochrome degradation. J Biol Chem 264: 4998–5005 (1989).
Jentsch S, Seufert W, Sommer T, Reins H-A: Ubiquitin-conjugating enzymes: Novel regulators of eukaryotic cells. Trends Biochem Sci 15: 195–198 (1990).
Johnson ES, Bartel B, Seufert W, Varshavsky A: Ubiquitin as a degradation signal. EMBO J 11: 497–505 (1992).
Joshi CP: Putative polyadenylation signals in nuclear genes of higher plants: a compilation and analysis. Nucl Acids Res 15: 9627–9640 (1987).
Kombrink E, Hahlbrock K: Responses of cultured parsley cells to elicitors from phytopathogenic fungi. Plant Physiol 81: 216–221 (1986).
Lam E, Benfey PN, Gilmartin PM, Fang R-X, Chua N-H: Site-specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc Natl Acad Sci USA 86: 7890–7894 (1989).
Lois R, Dietrich A, Hahlbrock K, Schulz W: A phenylalanine ammonia-lyase gene from parsley: Structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO J 8: 1641–1648 (1989).
Lozoya E, Block A, Lois R, Hahlbrock K, Scheel D: Transcriptional repression of light-induced flavonoid synthesis by elicitor treatment of cultured parsley cells. Plant J 1: 227–234 (1991).
Meier I, Hahlbrock K, Somssich IE: Elicitor-inducible and constitutive in vivo DNA footprints indicate novel cis-acting elements in the promoter of a parsley gene encoding pathogenesis-related protein 1. Plant Cell 3: 309–315 (1991).
Nover L: Heat Shock Response. CRC Press, Boca Raton, FL (1991).
Özkaynak E, Finley D, Solomon MJ, Varshavsky A: The yeast ubiquitin genes: a family of natural gene fusions. EMBO J 6: 1429–1439 (1987).
Özkaynak E, Finley D, Varshavsky A: The yeast ubiquitin gene: head-to-tail repeats encoding a polyubiquitin precursor protein. Nature 312: 663–666 (1984).
Pieterse CMJ, Risseeuw EP, Davidse LC: An in planta induced gene of Phytophtora infestans codes for ubiquitin. Plant Mol Biol 17: 799–811 (1991).
Pollman L, vonKampen J, Wettern M: Ubiquitin in a lower plant: Characterization of ubiquitin-encoding DNA and RNA from Chlamydomonas reinhardtii. Eur J Biochem 202: 197–204 (1991).
Pollman L, Wettern M: The ubiquitin system in higher and lower plants—pathways in protein metabolism. Bot Acta 102: 21–30 (1989).
Sambrook I, Fritsch EF, Maniatis T, Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1989).
Sanger F, Nicken S, Coulson AR: DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467 (1977).
Schlesinger MJ, Bond U: Ubiquitin genes. Oxford Surv Eukaryotic Genes 4: 77–91 (1987).
Schulze-Lefert P, Dangl JL, Becker-André M, Hahlbrock K, Schulz W: Inducible in vivo DNA footprints define sequences necessary for UV light activation of the parsley chalcone synthase gene. EMBO J 8: 651–656 (1989).
Shanklin J, Jabben M, Vierstra RD: Red light-induced formation of ubiquitin-phytochrome conjugates: Identification of possible intermediates of phytochrome degradation. Proc Natl Acad Sci USA 84: 359–363 (1987).
Sharp PM, Li W-H: Ubiquitin genes as a paradigm of concerted evolution of tandem repeats. JME 25: 58–64 (1987).
Sheen J-Y, Seed B: Electrolyte gradient gels for DNA sequencing. Bio Techniques 6: 942–944 (1988).
Singh K, Dennis ES, Ellis JG, Llewellyn DJ, Tokuhisa JG, Wahleithner JA, Peacock WJ: OCSBF-1, a maize ocs enhancer binding factor: Isolation and expression during development. Plant Cell 2: 891–903 (1990).
Somssich IE, Bollmann J, Hahlbrock K, Kombrink E, Schulz W: Differential early activation of defense-related genes in elicitor-treated parsley cells. Plant Mol Biol 12: 227–234 (1989).
Somssich IE, Schmelzer E, Bolmann J, Hahlbrock K: Rapid activation by fungal elicitor of genes encoding ‘pathogenesis related’ proteins in cultured parsley cells. Proc Natl Acad Sci USA 83: 2427–2430 (1986).
Somssich IE, Schmelzer E, Kawalleck P, Hahlbrock K: Gene structure and in situ transcript localization of pathogenesis-related protein 1 in parsley. Mol Gen Genet 213: 93–98 (1988).
Swindle J, Ajioka J, Eisen H, Sanwal B, Jaquemot C, Browder Z, Buck G: The genomic organization and transcription of the ubiquitin genes of Trypanosoma cruzi. EMBO J 7: 1121–1127 (1988).
Trezzini GF, Horrichs A, Somssich IE: Isolation of putative defense-related genes from Arabidopsis thaliana and expression in fungal elicitor-treated cells. Plant Mol Biol (in press).
van deLöcht U, Meier I, Hahlbrock K, Somssich IE: A 125 bp promoter fragment is sufficient for strong elicitor-mediated gene activation in parsley. EMBO J 9: 2945–2950 (1990).
Walter MH, Hahlbrock K: Synthesis of characteristic proteins in nutrient-depleted cell suspension cultures of parsley. Planta 166: 194–200 (1985).
Weisshaar B, Armstrong GA, Block A, da Costa e Silva O, Hahlbrock K: Light-inducible and constitutively expressed DNA-binding proteins recognizing a plant promoter element with functional relevance in light responsiveness. EMBO J 10: 1777–1786 (1991).
Wiebauer K, Herrero J-J, Filipowicz W: Nuclear pre-mRNA processing in plants: Distinct modes of 3′-splicesite selection in plants and animals. MCB 8: 2042–2051 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawalleck, P., Somssich, I.E., Feldbrügge, M. et al. Polyubiquitin gene expression and structural properties of the ubi4-2 gene in Petroselinum crispum . Plant Mol Biol 21, 673–684 (1993). https://doi.org/10.1007/BF00014550
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00014550