Abstract
Since extracorporeal membrane oxygenator (ECMO) has been utilized to save countless lives by providing continuous extracorporeal breathing and circulation to patients with severe cardiopulmonary failure. In particular, it has played an important role during the COVID-19 epidemic. One of the important composites of ECMO is membrane oxygenator, and the core composite of the membrane oxygenator is hollow fiber membrane, which is not only a place for blood oxygenation, but also is a barrier between the blood and gas side. However, the formation of blood clots in the oxygenator is a key problem in the using process. According to the study of the mechanism of thrombosis generation, it was found that improving the hemocompatibility is an efficient approach to reduce thrombus formation by modifying the surface of materials. In this review, the corresponding modification methods (surface property regulation, anticoagulant grafting, and bio-interface design) of hollow fiber membranes in ECMO are classified and discussed, and then, the research status and development prospects are summarized.
Graphical abstract

Similar content being viewed by others
1 Introduction
Extracorporeal membrane oxygenation (ECMO) is an advanced medical equipment, which acts a vital role in the treatment of critically ill patients with cardiopulmonary impairment caused by infectious diseases such as “New Coronavirus”, “H1N1”, and “SARS” or other causes. Artificial lung has been applied in clinic for the first time since 1953. The initial use of artificial lung was limited to cardiopulmonary bypass (CPB) in cardiac surgery [1]. In 1972, ECMO was derived from traditional extracorporeal CPB technology and used to cure severe acute respiratory distress syndrome (ARDS) [2,3,4]. Great progress has been obtained in the fields of internal medicine, pediatrics, intensive care, and transplantation [5].
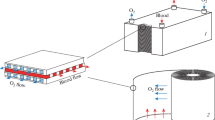
Since the end of 2019, humans have been concerned about the medical treatment of critically ill patients in the outbreak of Corona Virus Disease 2019 (COVID-19) [6]. ECMO has been very effective in the therapy process, because novel coronavirus attacks alveolar cells and causes the lungs to lose ventilation and then patients cannot normally breathe. ECMO medical equipment is used to replace the patient’s natural lung function in emergency process. As shown in Fig. 1a, the main principle of ECMO is that as blood passes through the oxygenator, the oxygen (O2) concentration increases and the carbon dioxide (CO2) concentration decreases [7, 8]. After the ECMO cycle, the non-oxygenated blood turns into oxygenated blood [9]. And then, it can be pumped back into the vein (VV access) or back into the artery (VA access) [5, 10]. This kind of machine provides continuous extracorporeal respiration and circulation for patients with severe cardiopulmonary failure, thus extending treatment time and reducing deaths due to severe hypoxia or CO2 retention. Nowadays, as the core facility for the treatment of severe cardiopulmonary failure, ECMO is also known as the “last straw” and “golden weapon” for severe patients.
The essence of ECMO is a modified artificial heart-lung machine, mainly composed of membrane oxygenator and blood pump [11, 12]. The former is mainly used to realize external respiratory, while the latter can maintain external respiration and replace the work of the heart because the blood pump has a heart-like pumping function [13, 14]. As shown in Fig. 1b, when a patient’s lung function is seriously impaired and conventional treatment fails, membrane oxygenator can undertake the task of gas exchange and gain precious time for the recovery of patients [15]. Similarly, when the cardiac function of patients is seriously damaged, the blood pump can perform the heart pumping function to achieve blood circulation.
The early artificial lung is to spread the blood on the plane to form a blood membrane and contact with oxygen to achieve the purpose of gas exchange and the later development of the bubble type artificial lung is to directly transfer oxygen into the blood for gas exchange [16, 17]. Both of them are to make oxygen and blood contact directly, which can cause damage to the blood to a certain extent, and also easily lead to gas embolism [18]. Nowadays, the separation membrane oxygenator is widely utilized in the artificial lung, and the use form has also developed from the original drum type and flat type to the hollow fiber membrane type [19, 20]. Since the late 1950s, the research on membrane materials with high permeability, high mechanical strength, free defects, and high biocompatibility has never stopped [13]. Membrane oxygenator which carries blood oxygen and function is the core component of ECMO system, so it is very vital to study the design, fabrication, and further modification of membrane.
In this paper, it is reviewed that the common types of hollow fiber membranes are applied for ECMO, and the influence of various natural or synthetic polymer biomaterials on the membrane properties is discussed and summarized. However, problems such as protein deposition, blood leakage, thrombosis, and hemolysis have appeared in the practical application of ECMO. Therefore, the membrane materials applied for blood oxygenation need to meet a series of requirements such as high-efficiency biocompatibility and hydrophobicity. Surface modification of membrane materials is one of the most important investigation strategies in the design and development of ECMO. This paper introduced and discussed the mechanism of thrombosis on the polymer materials surface and the modification methods of biomaterials surface to enhance their blood compatibility. Finally, some directions and views on the future modification methods of ECMO membrane materials are proposed.
2 Polymeric hollow fiber membranes for ECMO
The gas permeable membrane is one of the main components of the ECMO unit, which is a barrier between blood and sweep gas. Silicone rubber has good oxygen permeability and blood compatibility, but its function of discharging carbon dioxide is poor. Nowadays, polymeric hollow fiber membranes are mostly applied for the membrane oxygenator in the ECMO system, in which basic structural characteristics are uniform pore size and high porosity. The pore structure of hollow fiber membranes has an extremely important effect on gas permeability and leakage of blood [21]. Inappropriate pore size or pore distribution will affect the realization of hollow fiber membrane oxygenation function.
Too large pore size causes blood leakage, while too small pore size causes a decrease in gas transmission capacity. The oxygenation effect is poor when the pore size distribution is uneven or the ratio is too small [18]. Various natural or synthetic polymeric biomaterials are widely introduced in the manufacture of biomedical materials, but the most of employed polymers are still traditional materials, such as cellulose [22], chitosan (CS) [23], polyethylene (PE) [24], polydimethylsiloxane (PDMS) [25,26,27,28], polyethersulfone (PES) [29,30,31], polysulfone (PSU) [32], and the combination of acrylate and polyurethane-silicone polymer carbon compound [33]. Highly hydrophilic materials such as ethyl cellulose arouse the leakage of blood, which greatly shortens its service life. Therefore, materials such as polyethylene with stronger mechanical strength and hydrophobicity are used to replace the hydrophilic materials to solve this problem. However, the transition to hydrophobic materials initially had the limitation of low O2 and CO2 transport speed. This problem has been partially solved by adding a highly oxygen-permeable hydrophobic polyorganosilane film [34].
For polydimethylsiloxane (PDMS), the coefficient of oxygen permeability P(O2) is 600 Barrer, while it is 20 Barrer for ethyl cellulose, 1 to 8 Barrer for polyethylene [35]. However, the oxygen permeability for PDMS membranes with different thicknesses of the continuous layer varies from 35 × 1010 to 90 × 1010 Barrer [16].
Although PDMS-based materials are the most widely utilized materials [36] in the preparation of ECMO membranes, they possess a series of major shortcomings. For example, the hydrophobic properties of these membranes lead to serious protein adhesion and thrombosis, so researchers will increase biocompatibility by changing the physico-chemical performance of the membrane surface, such as surface hydrophilic modification, which will have a certain influence on the membrane performance [37,38,39,40].
Polypropylene (PP), composed of –CH2CH(CH3)– repeating units, is a linear hydrocarbon polymer [41]. It is the classical utilized polymer for microporous membrane preparation [42, 43]. PP membranes are not only widely used in water treatment, gas separation, and membrane crystallization, but also applied in the biomedical fields such as hemodialysis, blood separation, blood oxygen exchanger, and biosensor [44]. Many hydrophilic modification methods are adopted to realize the functionalized PP membranes, including physical modification, graft polymerization, and plasma treatment. In a blood oxygenator application, platelet adhesion and protein adsorption are occurrence on the unmodified PP hollow fiber membrane surface. The products of Terumo Group in Japan (X-Coating) are coated with zwitterionic on the surface of PP material to make the material show hydrophilic properties and thus reduce surface protein fouling. However, PP membrane is prone to blood leakage defects during use [42].
The research of poly(4-methyl-1-pentene) (PMP) membrane has realized the integration of the microporous internal structure and the dense outer layer in contact with blood, which is the most important recent practical achievement in the field of ECMO membrane materials [13]. Hollow fiber PMP oxygenator has excellent performance in gas exchange and maximum solves the problem of PP hollow fiber membrane blood leakage. The blood flow resistance is relatively low and it is easier to start. There are different kind of PMP membrane oxygenators currently commercially available, including Hilite LT (Medos), Lilliput 2 (Sorin), Quadrox-ID (Maquet), and so on [11]. As shown in Fig. 2, compared with other polymers, the oxygen transmission rate (OTR) of PMP has a great advantage [45,46,47]. And Table 1 summarizes the development process of ECMO dedicated gas-blood exchange membrane.
3 Hemocompatibility modification of ECMO membrane materials
Compared with other medical devices, ECMO has larger blood contact area. The surface area of oxygenator contacting blood ranges from 0.8 to 2.5 m2, and the volume reaches 75 to 250 ml. When the blood contacts with the surface of any non-autogenous materials, a series of reactions will occur rapidly, which will affect the human coagulation system and immune system, and eventually lead to thrombosis or hemolysis. Therefore, it is necessary to promote the biocompatibility by systemic anticoagulation or adjusting the physical and chemical characteristics of the material surface, which is huge challenges in clinical application of ECMO, especially in the term of lung transplantation or lung recovery. Bleeding occurs in 27% to 60% of adult patients, and thrombosis occurs in approximately 10% of patients [48]. Thrombus is composed of platelets, red blood cells and fibrin network on the surface. Cells cannot adhere to the polymeric membrane directly, which need the receptor to bind with the protein on the membrane surface. Hence, the occurrence of coagulation and inflammation in ECMO is the adsorption of plasma proteins (covalent or ionic reactions) [49]. As the plasma protein adsorption on the material surface, platelet adhesion to the surface of the plasma protein and complete their activation, platelet activation after release tissue factors (TF), which can promote the generation of thrombin and further activate platelet, finally fibrin and platelets and red blood cells on the material surface to form the thrombus and accumulating expand [50, 51]. The cell-based model can accurately describe the enzyme cascade, and more clearly express the interaction between the inflammatory system and the coagulation system (Fig. 3), which are interrelated, and the activation of one system tends to cross-activation of the other. According to the cell coagulation model, in order to alleviate the formation of thrombus, it can be intervened in two aspects roughly: (1) reduce the adhesion of fibrin on the material surface and (2) block or reduce the activation of thrombin. For the thrombosis pathway, several modification strategies can be proposed as shown in Fig. 4. The modification of membrane surface is very important to reduce the harmful phenomena such as thrombus.
Cell-based coagulation model [49]. In the cell-based model of coagulation, three phases of coagulation take place on the surface of cellular elements of the blood. While platelets take center stage, the impact of TF-bearing cells cannot be overlooked because multiple cell types can express TF, including endothelial cells, monocytes, and macrophages. Inactive forms of the coagulation factors are presented in blue ovals while activated versions are presented in red ovals. Coagulation complexes are enclosed in parallelograms or hexagons. Drugs that interact with the coagulation cascade are identified and point to the specific factors they inhibit
Nowadays, the top three suppliers in the global ECMO market: The first is Germany’s Maquet, which occupies more than 70% of the Chinese market, followed by Medtronic at 10%. In addition, Sorin accounts for 10%. Other manufacturers are Terumo, Medos, and so on. The material modification methods of each company are summarized in Table 2. It can be found that the most commonly adopted modification approaches can be classified as chemical modification and biological modification, chemical modification by grafting or coated polymer to change membrane surface properties or grafting heparin to reduce the activation of thrombin. Biological modification is through the utilization of albumin and phosphoryl choline build imitation biological cell membrane interface in the material surface. Combined with the relevant literature, the modification methods of membrane materials have always been concerned by researchers, and the utilized modification approaches are also very extensive [52,53,54,55,56]. In this review, we analyze and summarize those methods; the modification strategies currently used were roughly classified into the following three types:
-
1.
Change the physicochemical properties of the membrane surface to obtain relatively inert in response to blood;
-
2.
Graft anticoagulant onto the membrane surface;
-
3.
Design the biomimetic membrane interface.
Various methods of surface modification to enhance blood compatibility will be discussed. In this review, the representative methods of polymer surface modification are described in detail according to the above classification.
4 Physicochemical modification of the membrane surface
By changing the physical and chemical performance of membrane surface, the reaction between the membrane material surface and blood can be reduced. According to different principles, modification methods include hydrophilic modification, superhydrophobic modification, and so on. Hydrophilic modification is a widely used modification method [37, 62, 63]. As shown in Fig. 5a, after hydrophilic modification, the surface of the material forms a hydration layer, and protein does not directly contact with membrane surface. A great deal of protein adsorbed on the membrane surface decreases, followed by a decrease in platelet adhesion and activation, thus reducing the generation of thrombus. In contrast to surface hydrophilicity, surface superhydrophobic structure uses the interface with very low surface energy to make the interface tension between solid and liquid equal to that between gas and liquid under such a state [64, 65]. As shows in Fig. 5b, the interpretation of superhydrophobic surface is that the contact of liquid droplets on the rough surface is a composite contact. The liquid droplets cannot fill the grooves on the rough surface because of the trapped air under the liquid droplets. The blood compatibility of superhydrophobic structure has been reported. The superhydrophobic structure has excellent self-cleaning and anti-corrosion characteristics; therefore, it possesses very low adhesion to protein substances in blood. Correspondingly, there is still a lack of literature support for the research on the durability of superhydrophobic structure characteristics in long-term continuous contact with water. There are many approaches to regulate the surface properties of ECMO membranes, such as chemical grafting, physical adsorption, ion deposition, etc., as following discussed.
4.1 Surface chemical modification
Grafting polymer onto the surface by chemical methods is the most common approaches in material modification. Initially, the researchers conducted a series of hydrophobic modification studies to reduce blood leakage, including poly (L-lactide) (PLLA), poly (choline phosphate methacrylate) copolymers, and poly (2-methoxyethyl acrylate) (PMEA) [67]; however, hydrophobic surface can aggravate protein adhesion fouling [68]. Hydrophilic modification is an effective method to enhance the biocompatibility, such as polyethylene oxide (PEO) or poly (ethylene glycol) (PEG), which are employed to enhance the surface hydrophilicity of materials and achieve antifouling ability [69,70,71]. Because of hydrophilic properties of PEO, it can interact with water to generate a hydration layer, which repels proteins [72]. Meanwhile, the configurational migration of the PEO polymer backbone also fabricates a large repulsive volume, making it spatially repel protein adhesion and blocking potential adsorption sites. The extensive application of PEO in antifouling is due to its biocompatibility and oxidation stability [73]. In fact, when PEO is utilized to the surface of model substrates with physically stable (e.g., silicon [74, 75], glass [76], and gold [77]), protein resistance has been established. In this case, the silicone resin can be directly grafted and batch modified, and the PEO chain can be stably maintained on the film surface, which is independent of environmental changes (air or water) [78].
In addition, there are also many studies using zwitterionic modification, including carboxyl betaine, betaine sulfate, and betaine phosphate, etc. [79]. Zwitterionic polymer is a kind of polymer that maintains neutral charge, with negative and positive charges within the same side chain, and has many modification applications in the blood contact materials. Therefore, zwitterionic polymer-modified materials exhibited excellent resistance to non-specific bacterial adhesion, platelet adhesion, and protein adsorption. For example, just as Fig. 6 shows, Zhao et al. selected poly[(2-(methacryloxy) ethyl)] carboxybetaine (poly(CABA)) ester monomer covered in surface and introduced this monomer on the polypropylene nonwoven fabric membrane (PP NWF) surface to realize hemocompatibility modification by the technique of plasma pretreatment and a UV-induced graft polymerization [80]. Gu et al. prepared functional PSU membrane with zwitterionic sulfobetaine, which has better antifouling performance and stability [32]. Bao et al. synthesized an amphiphilic random copolymer composed of 2-methacryloyloxyethyl phosphorylcholine (MPC), butyl methacrylate (BMA), and trimethoxysilyl propyl methacrylate (TSMA) [81]. The copolymer has zwitterionic phosphorylated choline group and hydrophobic butyl side chain, which can be adsorbed on the hydrophobic surface to generate an outer membrane simulation coating.
4.2 Physical adsorption modification
In general, the chemical modification process is complex, and toxic monomer residues are often mixed; therefore, physical adsorption modification is sometimes selected [82]. Because van der Waals force generates between any two molecules, physical adsorption can occur on any solid surface. The molecules on the adsorbent surface have a free force field to attract the adsorbate due to the unbalanced force. Because it is caused by the adsorption between molecules, including the weak binding force, the low adsorption heat, and the rapid adsorption and desorption speed. To a certain extent, adsorbed substances are easy to resolve; therefore, physical adsorption is reversible.
The most difficult point of using physical dip-coating instead of chemical grafting is the stability of the coating [83]. Due to the lack of stability, physical dip-coating must be mixed with some chemicals which are easy to adhere to the substrate, such as surface-modifying additives (SMAs); it was an amphiphilic coupling polymer. For example, SMAs can be selected to modify polyurethane (PU) membrane, by spraying a stable SMA and poly (ether-urethane) on the outer wall of the membrane; the anticoagulation performance of PU membrane surface is finally realized and the stability was ensured [84]. Amiji and Park also reported that PEO homopolymer and block copolymer were physically adsorbed on the biomaterial surface to prepare PEO-modified surface, and further enhance the blood compatibility of biomaterials [85]. Additionally, some simple physical approaches (e.g., flame and corona discharge) are chosen to apply at the industrial level, as shown in Fig. 7.
a Schematic diagram of hydrophilic modification. b Surface roughness for surface wetting models [66]
4.3 Plasma deposition
Plasma chemical vapor deposition (PCVD) is a technology that uses plasma to activate reactive gases and promote them to chemically react on the substrate surface or near-surface space to form solid films [87]. The basic principle of PCVD technology is under the action of high frequency or direct current electric field, and then, the source gas is ionized to form plasma. Using low-temperature plasma as energy source, a proper amount of reaction gas is introduced, and plasma discharge is used to activate the reaction gas and realize chemical vapor deposition; the flow is shown in the Fig. 8. The advantage of PCVD is that the collision between electrons and gas molecules can promote the breaking and recombination of chemical bonds of reaction gas molecules, leading to the generation of more active chemical groups, while the whole reaction system maintains a low temperature [86].
Schematic illustration of the procedure for the preparation of the poly(CABA)-modified NWF membrane [80]
Pulsed vacuum cathode arc plasma deposition technology has been introduced to coat the poly (4-methyl-1-pentene) (PMP) gas exchange membrane with titanium dioxide to form a stable intermediate layer, which provides conditions for subsequent adhesion of proteins to the PMP membrane with strong hydrophobicity [88]. PCVD can also form amine functional groups on the surface of PMP fibers, and then bind functional amphoteric polymers (such as sulfonate betaine) to the material surface through ammonium functional groups [89]; the modified PMP membrane showed the decrease of 80–95% in platelet deposition from whole ovine blood in comparison with non‐modified PMP membrane [90]. However, the energy consumption of PCVD technology is higher than that of other chemical modification methods.
4.4 Self-assembly technology
Layer by layer (LbL) assembly has gradually become a common approach to fabricate multilayer films on the surface of materials [91]. Multilayer films are generally composed of non-covalent electrostatic. Due to the salt structure of zwitterionic polymers, multilayer zwitterionic polymers are difficult to fabricate by ordinary non-covalent electrostatic interactions. In addition, under the influence of different environments, covalent cross-linked multilayers are more stable than non-covalent electrostatically assembled multilayers. 3,4-Dihydroxyphenylalanine (DOPA) and other catechol composite can be easily self-assembled in alkaline aqueous mixtures to form crosslinked polydopamine (PDA) structure and can strongly bind to different substances [92, 93]. Zhao and co-workers prepared cross-linked zwitterionic polymer multilayer and citric acid composite multilayer on PSU membrane through click chemistry enabled LbL assembly [94]. The composite multilayer membrane has resistance to platelet adhesion and protein adsorption and leads to more blood coagulation time. As shown in Fig. 9, on the paclitaxel/chitosan (PTX/CS) NF membrane, the PMA polyanion and CS polycation were performed by layer-by-layer (LbL) assembly. The hemocompatibility of modified membrane surface was importantly enhanced, as shown by 60–70% adsorption suppression for bovine serum albumin (BSA) and bovine plasma fibrinogen and 94% adhesion suppression for platelets [95].
a Principles of corona treatment. b Principles of flame treatment [86]
4.5 Design membrane surface structure
Nowadays, it has been studied that the bio-adhesion behavior could be adjusted on the chemical gradient surface [96, 97]. Some reports have also shown that the gradient surface of the material has good hemocompatibility and can reduce thrombosis, which further means that due to the spontaneous droplet movement of the gradient surface, it may be utilized to modify the material surface to alleviate thrombosis [98, 99]. So far, many methods have been investigated to obtain gradient surfaces, including the creation of chemical gradients, structural morphologies, temperature, and power gradients [100, 101]. The creation of surface chemistry and structural terrain gradients is of increasing interest due to the reduction in external energy supply.
For example, PDMS surfaces can be prepared by single-step laser treatment, or on this basis, Zhang et al. also combine the adhesion resistance, flow resistance, and hemolysis rate of the wettability gradient surface to improve the performance of the membrane surface. As can be seen in Fig. 10, a wettability gradient surface was fabricated via ultra-short pulse laser etching technology, and then coated with stearic acid [102]. After treatment, the surface could form a superhydrophobic self-cleaning interface, which effectively reduces the fouling of blood on the material surface. It can also be done by simple diffusion and adsorption; an alkyl trichlorosilane (DTS) paraffin oil solution was used to form a wettability gradient surface on the silicon surface [100]. However, the membrane surface may lose its super-hydrophobic property easily due to immersion in long-term aqueous solution [103]; based on the current investigation, it is still difficult to meet the requirements of practical application.
5 Graft anticoagulant
Anticoagulants are used to prevent and treat diseases of endovascular embolism or thrombosis. It can be divided into antiplatelet drugs and anticoagulant agents [104]. Antiplatelet drugs can inhibit the metabolism of arachidonic acid (AA) and increase the concentration of adenosine cyclic phosphate (cAMP), thereby inhibiting platelet adhesion and aggregation. Common types include aspirin, dipyridamole, clopidogrel, etc. Anticoagulants can avoid blood clotting by affecting certain coagulation factors during the coagulation process, including heparin, low molecular weight heparin, Warfarin, Rivaroxaban, Dabigatrine etidic acid, and Agratoban, etc. [104]. The action mechanism and dose type of common anticoagulants are shown in Table 3. Anticoagulants used for injection usually can be directly conducted to modify the surface of membrane materials. Figure 11 summarizes the specific blocking sites of major anticoagulants in the market during the coagulation process. Grafting anticoagulant on the basis of polymer modification to enhance the blood compatibility of polymer is a vital direction of surface modification of ECMO membrane materials [105].
The specific composition of each layer in LbL coating [95]
5.1 Heparin
Heparin is a kind of widely used, powerful anticoagulant drugs [107]. As can be seen from Fig. 11, heparin is a highly effective natural systemic anticoagulant, which is mainly used to prevent and treat thrombosis or embolism diseases, such as myocardial infarction, thrombophlebitis, pulmonary embolism, etc., and also used in hemodialysis, extracorporeal circulation, catheterization, microvascular surgery, and other operations. It relies on antithrombin III (AT III) to inhibit thrombin, by changing the AT III conformation, increased its attached to the ability of thrombin. Therefore, heparin can effectively inactivate thrombin and contains serine proteases (such as XII alpha factor, XI alpha factor, IX alpha factor, X alpha factor, etc.) [108].
Heparin immobilization is usually accomplished by surface covalent bonding and surface polymerization or surface ion interaction. Heparin contains acidic mucopolysaccharides of varying lengths, so one can add a layer of amino group on the surface, and then reacts with the end of the polysaccharide chain of heparin. The linking process is relatively simple and suitable for various compounds (Fig. 12). The end-point covalent bonding between polymer surface and heparin was first marketed in the form of Carmeda bioactive surfactant (Carmeda, Switzerland). Later, similar heparin coatings (such as Hilite LT (MEDOS) and Bioline (MAQUET)) have been used by other manufacturers. Generally, the application of heparin coating is considered to be successful in the medical field, but heparin will non-specifically bind other molecules, such as platelet factor 4 (PF4), plasma protein, and white blood cells, which not only decreases the availability of heparin binding, but leads to side effects such as thrombocytopenia [109]. It may further improve the risk of bleeding complications in patients with bleeding, and therefore, the use of heparin requires constant monitoring of activated partial thromboplastin time (APTT).
Schematic illustration of the fabrication process of wettability gradient surface [102]
5.2 Argatroban
Argatroban (AG), as a piperidine carboxylic acid derivative of the synthesized L-arginine, can directly inhibit the activity of thrombin reversibly [110]. Both free thrombin in circulation and thrombin in blood clots can combine with it rapidly to produce anticoagulant effect [111].
Many literatures have reported the use of AG in ECMO therapy for thrombocytopenia patients [106, 112]. In imitation of the idea of the surface of heparin modified material, researchers used AG to improve the anticoagulant energy on the material surface. AG was applied to coat polyurethane stent and found that the stent had good anticoagulant effect [113]. As shown in Fig. 13, Dai et al. proposed the strategy of using AG instead of heparin as coagulant to modify the surface of PES membrane, and use PDA as intermediate linker to graft AG to modify PES membrane surface can effectively reduce thrombocytopenia caused by heparin [29]. As we all know, AG is not absorbed in the gastrointestinal tract; therefore, it must be administered in parenteral way. However, this drug by intravenous injection can be toxic to the liver.
The specific blocking sites of anticoagulants during the coagulation process [106]
5.3 Bivalirudin
As a synthetic version of the leech-derived anticoagulant hirudin, bivalirudin (BVLD) has two thrombin binding sites [114, 115]. The remainder of the drug is cleared through a renal mechanism, so bivalirudin is more attractive than argatroban for patients with liver failure. Because of the partial enzymatic cleavage, bivalirudin has a short half-life (30 min), which is suitable for short-term treatments, such as extracorporeal circulation and percutaneous coronary intervention [49]. Although the drug degrades with stagnation of blood flow, it may cause unintended clotting in the extracorporeal circulation machine’s repository or in any stagnation region of the ECMO circuit. This property increases the difficulty of clinical use.
Fixing the Bivalirudin to the membrane material can further control the drug’s slow release and reduce the blood clotting caused by the drug during circulation. By introduce plasma polymerized allylamine coating or introduce a hydrophobic cap onto the membrane surface, then connect the BVLD to the membrane surface [116]. Or as shown in Fig. 14, Yang et al. used PDA to functionalize TiO2 nanotube arrays. Meanwhile, the BVLD was selected as a model drug. Modified membrane material both in vitro and ex vivo blood evaluation results confirmed that this coating was importantly enhanced the hemocompatibility [117]. However, only little corresponding work on covalent immobilization of BVLD on biomedical materials was reported for enhancing hemocompatibility, because the hydrophobic cap may prevent the hydrophilic drug release into an aqueous solution.
The scheme for the dopamine cross-linking on PE porous membranes and subsequent heparin immobilization [24]
6 Design biofilm biomimetic interface
Endothelial cells (ECs) constituted the inner wall of the blood vessel that are the interface between blood in the vascular lumen and other blood vessel walls (single-layer squamous epithelium) [118]. ECs are located along the entire circulatory system, from the heart to the smallest capillaries, located between the blood and the vascular tissue. It cannot only ensure the metabolic exchange of plasma and tissue fluid, but also synthesize and secrete a great deal of biologically active substances, so that it guarantees the normal contraction and relaxation of blood vessels [119]. The most important thing is to achieve a balance between coagulation and anticoagulation and preserve the normal flow of blood. And there are natural anticoagulant composites on the endothelial cell membrane (e.g., prostaglandin (PGI) and heparin).
Just as shown in Fig. 15b, anticoagulant material on the endothelial cell surface can lessen the formation of thrombosis and platelet activation. Endothelial change not only on the physical cover the thrombus cells and blood coagulation factor to the outer surface of the adhesive, but also by resisting thrombosis and the expression of surface of anti-inflammatory molecules inhibit thrombosis, actively stop the bleeding [120].
6.1 Surface endothelialization
Endothelialization on the anticoagulant material surface can decrease thrombus formation and platelet activation. It is considered to be an effective method to obtain complete compatibility between biological blood and materials; thrombosis is inhibited to actively maintain hemostasis through the expression of anti-thrombosis and anti-inflammatory surface molecules [121, 122]. As shown in Fig. 16, by establishing a continuous layer of endothelial cells on the PMP membrane surface, the blood compatibility of the membrane surface can be effectively improved.
PDA coating and AG/mPEG-NH2 immobilization onto the PES membrane surface [29]
Endothelialization of the surface can be obtained by two different approaches: in vitro pre-endothelialization of the EC or endothelial progenitor cells (EPCs)-based in vivo induced self-endothelialization [47]. In vitro endothelialization was initially developed by direct implantation of autologous endothelium into the luminal surfaces of synthetic vascular grafts, scaffolds, and tissue-engineered vessels prior to implantation. EPCs are mononuclear cells derived from bone marrow and have the potential to differentiate into mature functional ECs. They can form functional endothelium in vivo and act a significant role in vascular repair and re-endothelialization [123]. However, the complex structure of endothelial cells and the high difficulty operation of material endothelialization, the current research on how to establish a single layer of endothelial tissue on the gas exchange of ECMO equipment is not mature enough.
6.2 Graft phosphorylcholine
Because it is difficult to construct endothelial cells on the surface of the material, it is also an effective modification method to graft the components of biofilm on the material surface to imitate the biological interface. As shown in Fig. 15a, the theme structure of the membrane is the phospholipid molecular layer. Phosphorylcholine (PC) group is the hydrophilic part of phospholipid, widely exists in biofilms, and can be utilized as biomimetic part of materials [124, 125]. After the surface modification of PC layer, the material has been proved to have excellent anti-platelet adhesion and anti-protein adsorption performance [126, 127], coating PC on the PDMS surface to change surface properties (Fig. 17). The biomimetic synthetic phospholipid polymer, composed of MPC and phosphorylcholine group, has enhanced the surface characteristics of biomaterials. During the research, the base material can also be changed into PMP material, and a layer of PC is coated on the PMP hollow fiber membrane surface to prepare the PC fully coated oxygenator; the results show that the biocompatibility is effectively improved [90].
Scheme of the possible loading mechanisms of BVLD to the NTs [117]
6.3 Graft protein
Coating the material with natural or synthetic proteins to reduce fibrin adhesion and platelet activation is also a widely used modification method. Protein-coated methods have been used in many companies’ products such as Hilite LT (MEDOS), Bioline, and Safeline (MAQUET).
The first protein used to pre-coat the blood contact surface is albumin [128]. Using ionic or covalent bonding to the surface of the material, the aim of the albumin pre-coating is to supply a protein base layer that can delay or reduce the biological response to severely hydrophobic surfaces. The adsorbed albumin not only improves the hydrophilicity of the surface, but also offers a competitive protein for fibrin to replace the adhesion of fibrin. Albumin covalently attached to the surface (e.g., Safeline manufactured by Maquet) guarantees that it remains on the surface of the material without being replaced. Subsequent anticoagulation studies of this coating implied the concentration of surface fibrinogen and platelets will decrease in a short period of time. Some evidence indicated that it will reduce the activation of coagulation factors [129]. BSA is a kind of natural globulin and is often used in a modified protein, can go through grafting polymerization by multiphase light to acrylic acid grafted membranes, and fix the BSA on the surface (Fig. 18), its protein can significantly reduce pollution of the modified membrane surface, Fang et al. transplanted BSA into the same polyether sulfone membrane surface to improve its biocompatibility [130].
ECs established cell-cell and cell-substrate contacts on TiO2-coated PMP films [88]
Schematic drawing for protein adsorption and platelet adhesion at a CS-GA/water interface and b cell outer membrane mimetic phosphorylcholine/water interface [127]
The scheme of UV-irradiated surface graft polymerization of PAA and subsequent BSA immobilization [131]
7 Conclusion and prospect
ECMO has developed for several decades, and it is vital to human survival and development. The key point in its long-term application is to enhance the property of membrane materials, and oxygen exchange efficiency is a vital indicator for evaluating the property of membrane materials. After decades of research progress, it is gradually developed into hollow fiber membrane material prepared by PP or PMP, which can meet the requirements of oxygen exchange for blood oxygenation [132,133,134,135,136].
On the premise of ensuring the blood oxygen exchange of membrane materials, the blood biocompatibility of membrane materials determines the use time of membrane oxygenator. At present, commercial hollow fiber membrane materials are mainly surface inertia modification based on PP and PMP materials, or surface modification using anticoagulant drugs and biomimetic interface strategies. Considering the limitation of single modification to enhance the performance, the future research can combine two or more modification strategies to carry out multi-layer modification to further improve the material performance. Although now, there have been several manufacturers of products on the market, for patients, the shortage of equipment and the high cost of ECMO treatment still prevent many patients from receiving effective treatment. Oxygenator needs frequent replacement, resulting in that the treatment cost and the operation difficulty increases, but the modification conditions far cannot satisfy the needs of patients with long time use of the medical profession. Thus, for the scientific researchers, modification of membrane materials for long-term operation is still a big challenge.
Abbreviations
- ECMO:
-
Extracorporeal membrane oxygenation
- CPB:
-
Cardiopulmonary bypass
- ARDS:
-
Acute respiratory distress syndrome
- COVID-19:
-
Corona Virus Disease 2019
- VV access:
-
Vein blood back into the vein
- VA access:
-
Vein blood back into the artery
- CS:
-
Chitosan
- PE:
-
Polyethylene
- PDMS:
-
Polydimethylsiloxane
- PES:
-
Polyethersulfone
- PSU:
-
Polysulfone
- PP:
-
Polypropylene
- PMP:
-
Poly(4-methyl-1-pentene)
- OTR:
-
Oxygen transmission rate
- PLLA:
-
Poly (L-lactide)
- PMEA:
-
Poly (2-Methoxyethyl acrylate)
- PEO:
-
Polyethylene oxide
- PEG:
-
Poly (ethylene glycol)
- NWF:
-
Nonwoven fabric membrane
- MPC:
-
2-Methacryloyloxyethyl phosphorylcholine
- BMA:
-
Butyl methacrylate
- TSMA:
-
Trimethoxysilyl propyl methacrylate
- CABA:
-
Poly[(2-(methacryloxy) ethyl)] carboxybetaine
- SMAs:
-
Surface-modifying additives
- PU:
-
Polyurethane
- PCVD:
-
Plasma chemical vapor deposition
- LbL:
-
Layer by layer
- DOPA:
-
3,4-Dihydroxyphenylalanine
- PDA:
-
Polydopamine
- BSA:
-
Bovine serum albumin
- DTS:
-
Alkyl trichlorosilane
- AA:
-
Arachidonic acid
- cAMP:
-
Adenosine cyclic phosphate
- AT III:
-
Antithrombin III
- PF4:
-
Platelet factor 4
- APTT:
-
Activated partial thromboplastin time
- AG:
-
Argatroban
- BVLD:
-
Bivalirudin
- ECs:
-
Endothelial cell
- PGI:
-
Prostaglandin
- EPC:
-
Endothelial progenitor cell
- PC:
-
Phosphorylcholine
- TF:
-
Tissue factors
References
Baffes TG, Fridman JL, Bicoff JP et al (1970) Extracorporeal circulation for support of palliative cardiac surgery in infants. Ann Thorac Surg 10(4):354–363
Bartlett RH, Gazzaniga AB, Jefferies MR et al (1979) Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. J Extra Corpor Technol 11:26–41
Kolobow T, Gattinoni L, Tomlinson T et al (1977) The carbon dioxide membrane lung (CDML): a new concept. Trans Am Soc Artif Intern Organs 23(1):17–21
Arazawa DT, Oh HI, Ye SH et al (2012) Immobilized carbonic anhydrase on hollow fiber membranes accelerates CO2 removal from blood. J Membr Sci 403–404:25–31
Makdisi G, Wang IW (2015) Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 7(7):166–176
Henry BM (2020) COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med 8(4)
Schmidt M, Tachon G, Devilliers C et al (2013) Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intens Care Med 39(5):838–846
Schlanstein PC, Limper A, Hesselmann F et al (2018) Experimental method to determine anisotropic permeability of hollow fiber membrane bundles. J Membr Sci 546:70–81
Lie SA, Hwang NC (2019) Challenges of brain death and apnea testing in adult patients on extracorporeal corporeal membrane oxygenation-a review. J Cardiothor Vasc An 33:2266–2272
Mugford M, Elbourne D, Field D (2008) Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Db Syst Rev 3:1–17
Lequier L, Horton SB, McMullan DM et al (2013) Extracorporeal membrane oxygenation circuitry. Pediatr Crit Care Med 14:7–12
Fujiwara T, Nagaoka E, Watanabe T et al (2013) New generation extracorporeal membrane oxygenation with MedTech Mag-Lev, a single-use, magnetically levitated, centrifugal blood pump: preclinical evaluation in calves. Artif Organs 37(5):447–456
Valencia E, Nasr VG (2020) Updates in pediatric extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth 34(5):1309–1323
Zhang J, Nolan TDC, Zhang T et al (2007) Characterization of membrane blood oxygenation devices using computational fluid dynamics. J Membr Sci 288(1–2):268–279
Thiagarajan RR, Barbaro RP, Rycus PT et al (2017) Extracorporeal life support organization registry international report 2016. Asaio J 63(1):60–67
Lim MW (2006) The history of extracorporeal oxygenators. Anaesthesia 61(10):984–995
Matsuda N, Sakai K (2000) Blood flow and oxygen transfer rate of an outside blood flow membrane oxygenator. J Membr Sci 170:153–158
Wang F, Luo F (2008) Research advances of membrane materials in membrane oxygenator. Journal of Clinical Rehabilitative Tissue Engineering Research 12(10):1927–1930
Catapano G, Papenfuss HD, Wodetzki A et al (2001) Mass and momentum transport in extra-luminal flow (ELF) membrane devices for blood oxygenation. J Membr Sci 184:123–135
Kim GB, Kim SJ, Kim MH et al (2009) Development of a hollow fiber membrane module for using implantable artificial lung. J Membr Sci 326(1):130–136
Lund LW, Hattler BG, Federspiel WJ (1998) Is condensation the cause of plasma leakage in microporous hollow fiber membrane oxygenators. J Membr Sci 147:87–93
Zhang D, Karkooti A, Liu L et al (2018) Fabrication of antifouling and antibacterial polyethersulfone (PES)/cellulose nanocrystals (CNC) nanocomposite membranes. J Membr Sci 549:350–356
Mao C, Qiu Y, Sang H et al (2004) Various approaches to modify biomaterial surfaces for improving hemocompatibility. Adv Colloid Interfac 110(1–2):5–17
Jiang JH, Zhu LP, Li XL et al (2010) Surface modification of PE porous membranes based on the strong adhesion of polydopamine and covalent immobilization of heparin. J Membr Sci 364(1–2):194–202
Klein S, Hesselmann F, Djeljadini S et al (2020) EndOxy: Dynamic long-term evaluation of endothelialized gas exchange membranes for a biohybrid lung. Ann Biomed Eng 48(2):747–756
Zhu H, Li X, Pan Y et al (2020) Fluorinated PDMS membrane with anti-biofouling property for in-situ biobutanol recovery from fermentation-pervaporation coupled process. J Membr Sci 609:118225
Stamatialis DF, Papenburg BJ, Gironés M et al (2008) Medical applications of membranes: drug delivery, artificial organs and tissue engineering. J Membr Sci 308(1–2):1–34
Dabaghi M, Saraei N, Fusch G et al (2020) Microfluidic blood oxygenators with integrated hollow chambers for enhanced air exchange from all four sides. J Membr Sci 596:117741
Dai Y, Dai S, Xie X, Ning J (2019) Immobilizing argatroban and mPEG-NH2 on a polyethersulfone membrane surface to prepare an effective nonthrombogenic biointerface. J Biomater Sci Polym Ed 30(8):608–628
He M, Cui X, Jiang H et al (2017) Super-anticoagulant heparin-mimicking hydrogel thin film attached substrate surfaces to improve hemocompatibility. Macromol Biosci 17(2):1600281
Li L, Cheng C, Xiang T et al (2012) Modification of polyethersulfone hemodialysis membrane by blending citric acid grafted polyurethane and its anticoagulant activity. J Membr Sci 405–406:261–274
Gu S, Xia H, Du J et al (2016) Surface modification of polysulfones via one-pot ATRP and click chemistry: Zwitterionic graft complex and their hemocompatibility. Fiber Polym 17(2):161–165
Yu J, Brisbois E, Handa H et al (2016) The immobilization of a direct thrombin inhibitor to a polyurethane as a nonthrombogenic surface coating for extracorporeal circulation. J Mater Chem B 4(13):2264–2272
Evseev AK, Zhuravel SV, Alentiev AY et al (2019) Membranes in extracorporeal blood oxygenation technology. Membranes and Membrane Technologies 1(4):201–211
Robb WL (1968) Thin silicone membranes–their permeation properties and some applications. Ann NY Acad Sci 146:119–137
Dabaghi M, Saraei N, Fusch G et al (2019) An ultra-thin, all PDMS-based microfluidic lung assist device with high oxygenation capacity. Biomicrofluidics 13(3):034116
Cutiongco MF, Anderson DE, Hinds MT et al (2015) In vitro and ex vivo hemocompatibility of off-the-shelf modified poly(vinyl alcohol) vascular grafts. Acta Biomater 25:97–108
Czermak P, Walz M, Catapano G (2006) Analysis of in vitro continuous wet-dry CO2 removal with hydrophilic membranes from slowly flowing blood. J Membr Sci 283:89–96
He S, Jiang X, Li S et al (2020) Intermediate thermal manipulation of polymers of intrinsic microporous (PIMs) membranes for gas separations. AIChE J 66(10):e16543
Zhu B, Jiang X, Hesl, S (2020) Rational design of poly(ethylene oxide) based membranes for sustainable CO2 capture. J Mater Chem A 8(46):24233–24252
Kim SS, Lloyd DR (1991) Microporous membrane formation via thermally-induced phase separation. III. Effect of thermodynamic interactions on the structure of isotactic polypropylene membranes. J Membr Sci 64(1–2):13–29
Himma NF, Anisah S, Prasetya N et al (2016) Advances in preparation, modification, and application of polypropylene membrane. J Polym Eng 36(4):329–362
Gu B, Du Q, Yang Y (2000) Microporous hollow fiber membranes formed from blends of isotactic and atactic polypropylene. J Membr Sci 164:59–65
Mosadegh-Sedghi S, Rodrigue D, Brisson J et al (2014) Wetting phenomenon in membrane contactors-causes and prevention. J Membr Sci 452:332–353
Edinger F, Schneck E, Schulte C et al (2020) Comparison of the effect of membrane sizes and fibre arrangements of two membrane oxygenators on the inflammatory response, oxygenation and decarboxylation in a rat model of extracorporeal membrane oxygenation. BMC Cardiovasc Disor 20(1):294
Tazeen H, Varadharaju N, Kannan M (2019) Changes in gas transmission rate and water vapour permeability in PP and LLDPE nano composite films. Research Journal of Agricultural Sciences 10(2):334–337
Ontaneda A, Annich GM (2018) Novel surfaces in extracorporeal membrane oxygenation circuits. Front Med (Lausanne) 5:321
Mazzeffi MA, Tanaka K, Roberts A et al (2019) Bleeding, thrombosis, and transfusion with two heparin anticoagulation protocols in venoarterial ECMO patients. J Cardiothorac Vasc Anesth 33(5):1216–1220
Maul TM, Massicotte MP, Wearden PD (2016) ECMO biocompatibility: Surface coatings, anticoagulation, and coagulation monitoring. In: Extracorporeal membrane oxygenation: Advances in therapy 27–61
More RB, Haubold AD, Bokros JC (2013) Pyrolytic carbon for long-term medical implants. Biomater Sci Third Edition 209–222
Ahrens I, Lip GY, Peter K (2010) New oral anticoagulant drugs in cardiovascular disease. Thromb Haemostasis 104(1):49–60
Ren X, Feng Y, Guo J et al (2015) Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering application. Chem Soc Rev 44(15):5680–5742
Wang Y, Shen J, Yuan J (2016) Design of hemocompatible and antifouling PET sheets with synergistic zwitterionic surfaces. J Colloid Interf Sci 480:205–217
Jiang Y, Su Y, Zhao L et al (2017) Preparation and antifouling properties of 2-(meth-acryloyloxy) ethyl choline phosphate based polymers modified surface with different molecular architectures by ATRP. Colloid Surface B 156:87–94
Zhang S, Cao J, Ma N, You M, Wang X, Meng J (2018) Fast and facile fabrication of antifouling and hemocompatible PVDF membrane tethered with amino-acid modified PEG film. Appl Surf Sci 428:41–53
Wang H, Shi X, Gao A et al (2018) Heparin free coating on PLA membranes for enhanced hemocompatibility via iCVD. Appl Surf Sci 433:869–878
Palanzo DA, Zarro DL, Manley NJ et al (2001) Effect of Carmeda bioActive surface coating versus Trillium biopassive surface coating of the oxygenator on circulating platelet count drop during cardiopulmonary bypass. Perfusion 16:279–283
Preston TJ, Ratliff TM, Gomez D et al (2010) Modified surface coatings and their effect on drug adsorption within the extracorporeal life support circuit. J Extra Corpor Technol 42:199–202
Ask A, Holt MD, Smith ML (2006) In vivo comparison study of FDA-approved surface-modifying additives and poly-2-methoxyethyl acrylate circuit surfaces coatings during cardiopulmonary bypass. The Journal of The American Society of Extra-Corporeal Technology 38:27–32
Reser D, Seifert B, Klein M et al (2012) Retrospective analysis of outcome data with regards to the use of Phisio(R)-, Bioline(R)- or Softline(R)-coated cardiopulmonary bypass circuits in cardiac surgery. Perfusion 27(6):530–534
Khan NU, Al-Aloul M, Shah R et al (2008) Early experience with the Levitronix Centrimag device for extra-corporeal membrane oxygenation following lung transplantation. Eur J Cardiothorac Surg 34(6):1262–1264
Wang BL, Li L, Zheng YF (2010) A study of TaxC1-x coatings deposited on biomedical 316L stainless steel by radio-frequency magnetron sputtering. Appl Surf Sci 257:696–703
Yu DG, Jou CH, Lin WC et al (2007) Surface modification of poly(tetramethylene adipate-co-terephthalate) membrane via layer-by-layer assembly of chitosan and dextran sulfate polyelectrolyte multiplayer. Colloid Surf B 54(2):222–229
Khorasani MT, Mirzadeh H (2004) In vitro blood compatibility of modified PDMS surfaces as superhydrophobic and superhydrophilic materials. J Appl Polym Sci 91:2042–2047
Li H, Zhao X, Chu G et al (2014) One-step fabrication of a superhydrophobic polymer surface from an acrylic copolymer containing POSS by spraying. RSC Adv 4(107):62694–62697
Movafaghi S, Wang W, Bark DL et al (2019) Hemocompatibility of super-repellent surfaces: current and future. Mater Horiz 6(8):1596–1610
Obstals F, Vorobii M, Riedel T et al (2018) Improving hemocompatibility of membranes for extracorporeal membrane oxygenators by grafting nonthrombogenic polymer brushes. Macromol Biosci 18(3):1700359
An Z, Dai F, Wei C et al (2018) Polydopamine/cysteine surface modified hemocompatible poly(vinylidene fluoride) hollow fiber membranes for hemodialysis. J Biomed Mater Res B 106(8):2869–2877
Chen H, Brook MA, Sheardown H (2004) Silicone elastomers for reduced protein adsorption. Biomaterials 25(12):2273–2282
Chen H, Zhang Z, Chen Y et al (2005) Protein repellant silicone surfaces by covalent immobilization of poly(ethylene oxide). Biomaterials 26(15):2391–2399
Lee S, Vörös J (2005) An aqueous-based surface modification of poly(dimethylsiloxane) with poly(ethylene glycol) to prevent biofouling. Langmuir 21:11957–11962
Sefton MV, Gemmell CH, Gorbet MB (2012) Nonthrombogenic treatments and strategies. In: Biomater Sci 1488–1509
Browning MB, Cereceres SN, Luong PT et al (2014) Determination of the in vivo degradation mechanism of PEGDA hydrogels. J Biomed Mater Res A 102(12):4244–4251
Papra A, Gadegaard N, Larsen NB (2001) Characterization of ultrathin poly(ethylene glycol) monolayers on silicon substrates. Langmuir 17:1457–1460
Sofia SJ, Premnath V, Merrill EW (1998) Poly(ethylene oxide) grafted to silicon surfaces: grafting density and protein adsorption. Macromolecules 31:5059–5070
Jo S, Park K (2000) Surface modification using silanated poly(ethylene glycol)s. Biomaterials 21:605–616
Prime KL, Whitesides GM (1991) Self-assembled organic monolayers: model systems for studying adsorption of proteins at surfaces. Science 252:1164–1167
Ngo BKD, Lim KK, Stafslien SJ et al (2019) Stability of silicones modified with PEO-silane amphiphiles: impact of structure and concentration. Polym Degrad Stabil 163:136–142
Yue WW, Li HJ, Xiang T et al (2013) Grafting of Zwitterion from polysulfone membrane via surface-initiated ATRP with enhanced antifouling property and biocompatibility. J Membr Sci 446:79–91
Zhao J, Song L, Shi Q et al (2013) Antibacterial and hemocompatibility switchable polypropylene nonwoven fabric membrane surface. ACS Appl Mater Inter 5(11):5260–5268
Bao LL, Huang HQ, Zhao J et al (2014) Preparation and characterization of zwitterionic phospholipid polymer-coated poly(lactic acid) nanoparticles. J Biomater Sci Polym Ed 25(14–15):1703–1716
Goushki MN, Mousavi SA, Abdekhodaie MJ et al (2018) Free radical graft polymerization of 2-hydroxyethyl methacrylate and acrylic acid on the polysulfone membrane surface through circulation of reaction media to improve its performance and hemocompatibility properties. J Membr Sci 564:762–772
Tsai CC, Deppisch RM, Forrestal LJ et al (1994) Surface modifying additives for improved device-blood compatibility. Asaio J 40(3):619–624
Wang DA, Ji J, Gao CY et al (2001) Surface coating of stearyl poly(ethylene oxide) coupling-polymer on polyurethane guiding catheters with poly(ether urethane) film-building additive for biomedical applications. Biomaterials 22:1549–1562
Amiji MM (1997) Synthesis of anionic poly(ethylene glycol) derivative for chitosan surface modification in blood-contacting applications. Carbohyd Polym 32:193–199
Fabbri P, Messori M (2017) Surface modification of polymers. In: Modification of Polymer Properties 109–130
Chen JY, Leng YX, Tian XB et al (2002) Antithrombogenic investigation of surface energy and optical bandgap and hemocompatibility mechanism of Ti(Ta+5)O2 thin films. Biomaterials 23:2545–2552
Pflaum M, Kuhn-Kauffeldt M, Schmeckebier S et al (2017) Endothelialization and characterization of titanium dioxide-coated gas-exchange membranes for application in the bioartificial lung. Acta Biomater 50:510–521
Malkin AD, Ye SH, Lee EJ et al (2018) Development of zwitterionic sulfobetaine block copolymer conjugation strategies for reduced platelet deposition in respiratory assist devices. J Biomed Mater Res B 106(7):2681–2692
Pieri M, Turla OG, Calabro MG et al (2013) A new phosphorylcholine-coated polymethylpentene oxygenator for extracorporeal membrane oxygenation: a preliminary experience. Perfusion 28(2):132–137
Genzer J, Efimenko K (2000) Creating long-lived superhydrophobic polymer surfaces through mechanically assembled monolayers. Science 290:2130–2133
Lee H, Dellatore SM, Miller WM et al (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318:426–430
Goddard JM, Hotchkiss JH (2007) Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci 32(7):698–725
Xiang T, Wang R, Zhao WF et al (2014) Covalent deposition of zwitterionic polymer and citric acid by click chemistry-enabled layer-by-layer assembly for improving the blood compatibility of polysulfone membrane. Langmuir 30(18):5115–5125
Liu Y, Ma X, Zhou T et al (2019) Layer by layer assembled phosphorylcholine groups on paclitaxel/chitosan nanofibers coatings for hemocompatibility improvement. Surf Coat Technol 357:984–992
Wang T, Handschuh-Wang S, Yang Y et al (2014) Controlled surface chemistry of diamond/beta-SiC composite films for preferential protein adsorption. Langmuir 30(4):1089–1099
Lee JH, Khang G, Lee JW et al (1997) Platelet adhesion onto chargeable functional group gradient surfaces. J Biomed Mater Res 40(2):180–186
Ito Y, Nakamura S, Sugimoto N et al (2018) Turbulence activates platelet biogenesis to enable clinical scale ex vivo production. Cell 174(3):636–648
Fuchs G, Berg N, Broman LM et al (2018) Flow-induced platelet activation in components of the extracorporeal membrane oxygenation circuit. Sci Rep 8(1):13985
Chaudhury MK, Whitesides GM (1992) How to make water run uphill. Science 256:1539–1541
Malvadkar NA, Hancock MJ, Sekeroglu K et al (2010) An engineered anisotropic nanofilm with unidirectional wetting properties. Nat Mater 9(12):1023–1028
Zhang Q, Dong J, Peng M et al (2020) Laser-induced wettability gradient surface on NiTi alloy for improved hemocompatibility and flow resistance. Mater Sci Eng C 111:110847
Zhou F (2015) Antifouling Surfaces and Materials. Springer, Berlin Heidelberg New York Dordrecht London
Fan P, Gao Y, Zheng M et al (2018) Recent progress and market analysis of anticoagulant drugs. J Thorac Dis 10(3):2011–2025
Tatsumi E (2007) Artificial lungs: current state and trends of clinical use and research and development. J Artif Organs 10(1):1–5
Bijak M, Saluk J, Szelenberger R et al (2016) Popular naturally occurring antioxidants as potential anticoagulant drugs. Chem Biol Interact 257:35–45
Mangoush O, Purkayastha S, Haj-Yahia S et al (2007) Heparin-bonded circuits versus nonheparin-bonded circuits: an evaluation of their effect on clinical outcomes. Eur J Cardio-thorac 31:1058–1069
Momand J, Zambetti GP, Olson DC et al (1992) The mdm-2 oncogene product forms a complex with the p53 protein and Inhibits p53-mediated transactivation. Cell 69:1237–1245
Ahmed I, Majeed A, Powell R (2007) Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J 83(983):575–582
Okamoto S, Hijikata A, Kikumoto R et al (1981) Potent inhibition of thrombin by the newly synthesized arginine derivative No. 805. The importance of stereo-structure of its hydrophobic carboxamide portion. Biochem Bioph Res Co 101(2):440–446
Coppens M, Eikelboom JW, Gustafsson D et al (2012) Translational success stories: development of direct thrombin inhibitors. Circ Res 111(7):920–929
Menk M, Briem P, Weiss B et al (2017) Efficacy and safety of argatroban in patients with acute respiratory distress syndrome and extracorporeal lung support. Ann Intensive Care 7(1):82
Nishi S, Nakayama Y, Ishibashi-Ueda H et al (2014) Occlusion of canine aneurysms using microporous self-expanding stent grafts: long-term follow-up. Clin Neurol Neurosurg 122:34–41
Warkentin TEJBP, Haematology RC (2004) Bivalent direct thrombin inhibitors: hirudin and bivalirudin. Best Pract Res Cl Ha 17(1):105–125
Warkentin TE, Greinacher A, Koster A (2008) Bivalirudin. Thromb. Haemostasis 99(5):830–839
Yang Z, Tu Q, Maitz MF et al (2012) Direct thrombin inhibitor-bivalirudin functionalized plasma polymerized allylamine coating for improved biocompatibility of vascular devices. Biomaterials 33(32):7959–7971
Yang Y, Li X, Qiu H et al (2018) Polydopamine modified TiO2 nanotube arrays for long-term controlled elution of bivalirudin and improved hemocompatibility. ACS Appl Mater Inter 10(9):7649–7660
Dejana E (2004) Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Bio 5(4):261–270
Michiels C (2003) Endothelial cell functions. J Cell Physiol 196(3):430–443
Sumpio BE, Riley JT, Dardik A (2002) Cells in focus: endothelial cell. Int J Biochem Cell Biol 34:1508–1512
Koenneker S, Teebken OE, Bonehie M et al (2010) A biological alternative to alloplastic grafts in dialysis therapy: evaluation of an autologised bioartificial haemodialysis shunt vessel in a sheep model. Eur J Vasc Endovasc 40(6):810–816
Achneck HE, Jamiolkowski RM, Jantzen AE et al (2011) The biocompatibility of titanium cardiovascular devices seeded with autologous blood-derived endothelial progenitor cells. Biomaterials 32(1):10–18
Pang JH, Farhatnia Y, Godarzi F et al (2015) In situ endothelialization: bioengineering considerations to translation. Small 11(47):6248–6264
Zhou L, Tan GX, Ning CY (2014) Modification of biomaterials surface by mimetic cell membrane to improve biocompatibility. Front Mater Sci 8(4):325–331
Sheikhpour M, Barani L, Kasaeian A (2017) Biomimetics in drug delivery systems: A critical review. J Control Release 253:97–109
Ren X, Feng Y, Guo J et al (2015) Correction: surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem Soc Rev 44(15):5745
Huangfu PB, Gong M, Zhang C et al (2009) Cell outer membrane mimetic modification of a cross-linked chitosan surface to improve its hemocompatibility. Colloid Surf B 71(2):268–274
Mulvihill JN, Oberling AF, Cazenave JP (1990) Surface passivation by human albumin of plasmapheresis circuits reduces platelet accumulation and thrombus formation. Experimental and clinical studies. J Biomed Mater Res 24(2):155–163
Zimmermann AK, Weber N, Aebert H et al (2007) Effect of biopassive and bioactive surface-coatings on the hemocompatibility of membrane oxygenators. J Biomed Mater Res B 80(2):433–439
Fang B, Cheng C, Li L et al (2010) Surface modification of polyethersulfone membrane by grafting bovine serum albumin. Fibers Polym 11(7):960–966
Zhang C, Jin J, Zhao J et al (2013) Functionalized polypropylene non-woven fabric membrane with bovine serum albumin and its hemocompatibility enhancement. Colloid Surf B 102:45–52
Li J, Zhu LP, Xu YY et al (2010) Oxygen transfer characteristics of hydrophilic treated polypropylene hollow fiber membranes for bubbleless aeration. J Membr Sci 362(1–2):47–57
Wang YB, Gong M, Yang S et al (2014) Hemocompatibility and film stability improvement of crosslinkable MPC copolymer coated polypropylene hollow fiber membrane. J Membr Sci 452:29–36
Huang X, Wang W, Zheng Z et al (2016) Dissipative particle dynamics study and experimental verification on the pore morphologies and diffusivity of the poly (4-methyl-1-pentene)-diluent system via thermally induced phase separation: the effect of diluent and polymer concentration. J Membr Sci 514:487–500
Wilfart FM, McNeil MV, Haelssig JB et al (2020) Validation and optimization of a membrane system for carbon dioxide removal in anesthesia circuits under realistic patient scenarios. J Membr Sci 601:117887
Markova SY, Gries T, Teplyakov VV (2020) Poly(4-methyl-1-pentene) as a semicrystalline polymeric matrix for gas separating membranes. J Membr Sci 598:117754
Acknowledgements
The authors would like to express their appreciation for the financial support of the National Key R&D Program of China, the National Natural Science Foundation of China, the Jiangsu Provincial Department of Human Resources and Social Security, and the Materials-Oriented Chemical Engineering State Key Laboratory Program.
Funding
This work was supported by the financial support of the National Key R&D Program of China (2020YFC0862903), the National Key R&D Program of China (2017YFC0403702), the National Natural Science Foundation of China (51861135203), the Jiangsu Provincial Department of Human Resources and Social Security (JNHB-036), the Materials-Oriented Chemical Engineering State Key Laboratory Program (KL19-04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, T., He, J., Wang, Z. et al. Modification strategies to improve the membrane hemocompatibility in extracorporeal membrane oxygenator (ECMO). Adv Compos Hybrid Mater 4, 847–864 (2021). https://doi.org/10.1007/s42114-021-00244-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42114-021-00244-x