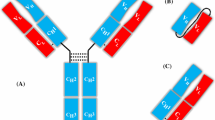
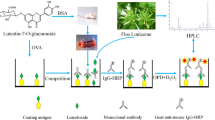
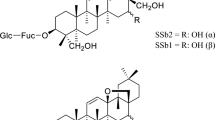
Abstract
Purpose of Review
This study summarizes the applications of monoclonal antibodies (MAbs) against bioactive natural compounds for the functional analysis of crude drugs.
Recent Findings
To date, we have established more than 40 kinds of MAbs against natural compounds and set up enzyme-linked immunosorbent assay (ELISA). The intracellular accumulation of harringtonine (HT) and its derivatives into cells was determined by ELISA using anti-HT MAb, and the uptake levels of these compounds conformed to their respective antiproliferative activities. Eastern blotting is an on-membrane quantitative analytical system that uses MAbs against natural compounds and it can also be used in a biological sample for pharmacokinetics study. Glycyrrhizin (GC) was detected in the serum of rats treated with GC by eastern blotting. Additionally, eastern blotting using MAb against 3-monoglucuronyl-glycyrrhetinic acid (3-MGA) could determine a potential causative agent of pseudoaldosteronism induced by excess intake of GC. The immunoaffinity column coupled with MAb made preparing an extract containing all components feasible except the target compound. Cell-based studies using GC-removed licorice extract, which was prepared by the immunoaffinity column conjugated with anti-GC MAb, revealed the potential functions of GC in licorice extract. Moreover, MAbs were used in determining cellular localization and target molecules of natural compounds. The combination of analysis using MAb and LC-MS/MS showed that aristolochic acid I (AA-I), a naturally occurring nephrotoxin and carcinogen, targets α-actinin-4 in the kidney cells.
Summary
MAbs against natural compounds are useful tools to determine real pharmacological functions, cellular localization, and molecular targets of natural compounds in crude extracts.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
WHO. WHO traditional medicine strategy 2014–2023. World Health Organization 2013.
Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–7. https://doi.org/10.1038/256495a0.
Sawada J, Janejai N, Nagamatsu K, Terao T. Production and characterization of high-affinity monoclonal antibodies against morphine. Mol Immunol. 1988;25(9):937–43. https://doi.org/10.1016/0161-5890(88)90133-2.
Weiler EW. Chemistry of plant protection. Berlin: Springer-Verlag; 1990. p. 145–220.
Cedeño-Arias M, Sánchez-Ramírez J, Blanco-Santana R, Rengifo-Calzado E. Validation of a flow cytometry based binding assay for evaluation of monoclonal antibody recognizing EGF receptor. Sci Pharm. 2011;79(3):569–81. https://doi.org/10.3797/scipharm.1104-18.
Tanaka H, Shoyama Y. Formation of a monoclonal antibody against glycyrrhizin and development of an ELISA. Biol Pharm Bull. 1998;21(12):1391–3. https://doi.org/10.1248/bpb.21.1391.
• Fujii S, Morinaga O, Uto T, Nomura S, Shoyama Y. Simultaneous determination of glycyrrhizin and liquiritin in licorice roots and Kampo medicines by combination enzyme-linked immunosorbent assay using anti-glycyrrhizin and anti-liquiritin monoclonal antibodies. J Immunoassay Immunochem. 2017;38(3):285–98. https://doi.org/10.1080/15321819.2016.1260586This article is one example of ELISA using MAbs against natural compounds.
Shan S, Tanaka H, Shoyama Y. Enzyme-linked immunosorbent assay for glycyrrhizin using anti-glycyrrhizin monoclonal antibody and an eastern blotting technique for glucuronides of glycyrrhetic acid. Anal Chem. 2001;73(24):5784–90. https://doi.org/10.1021/ac0106997.
Shoyama Y. Standardization of licorice and TCM formulations using eastern blot fingerprinting analysis. J Chemother. 2013:573070. https://doi.org/10.1155/2013/573070.
Fujii S, Morinaga O, Uto T, Nomura S, Shoyama Y. Development of double eastern blotting for major licorice components, glycyrrhizin and liquiritin for chemical quality control of licorice using anti-glycyrrhizin and anti-liquiritin monoclonal antibodies. J Agric Food Chem. 2016;64(5):1087–93. https://doi.org/10.1021/acs.jafc.5b04732.
Xu J, Tanaka H, Shoyama Y. One-step immunochromatographic separation and ELISA quantification of glycyrrhizin from traditional Chinese medicines. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;850(1–2):53–8. https://doi.org/10.1016/j.jchromb.2006.10.062.
Uto T, Morinaga O, Tanaka H, Shoyama Y. Analysis of the synergistic effect of glycyrrhizin and other constituents in licorice extract on lipopolysaccharide-induced nitric oxide production using knock-out extract. Biochem Biophys Res Commun. 2012;417(1):473–8. https://doi.org/10.1016/j.bbrc.2011.11.143.
Uto T, Tung NH, Morinaga O, Shoyama Y. Interaction analysis of glycyrrhizin on licorice extract-induced apoptosis of human leukemia cells by knockout extract. Nat Prod Chem Res. 2013;1:105. https://doi.org/10.4172/2329-6836.1000105.
•• Hsu YC, Chang PJ, Tung CW, Shih YH, Ni WC, Li YC, et al. De-glycyrrhizinated licorice extract attenuates high glucose-stimulated renal tubular epithelial-mesenchymal transition via suppressing the Notch2 signaling pathway. Cells. 2020;9(1):125. https://doi.org/10.3390/cells9010125This article is one example indicating that KO extract is a useful approach for determining the potential function of target natural compounds in extracts on biological studies.
Fujii S, Tuvshintogtokh I, Mandakh B, Munkhjargal B, Uto T, Morinaga O, et al. Screening of Glycyrrhiza uralensis Fisch. ex DC. containing high concentrations of glycyrrhizin by eastern blotting and enzyme-linked immunosorbent assay using anti-glycyrrhizin monoclonal antibody for selective breeding of licorice. J Nat Med. 2014;68(4):717–22. https://doi.org/10.1007/s11418-014-0847-7.
•• Morinaga O, Ishiuchi K, Ohkita T, Tian C, Hirasawa A, Mitamura M, et al. Isolation of a novel glycyrrhizin metabolite as a causal candidate compound for pseudoaldosteronism. Sci Rep. 2018;8(1):15568. https://doi.org/10.1038/s41598-018-33834-9This article shows that analysis using the eastern blotting determined a new marker compound causing an adverse effect.
•• Ishiuchi K, Morinaga O, Ohkita T, Tian C, Hirasawa A, Mitamura M, et al. 18β-Glycyrrhetyl-3-O-sulfate would be a causative agent of licorice-induced pseudoaldosteronism. Sci Rep. 2019;9(1):1587. https://doi.org/10.1038/s41598-018-38182-2This article shows that analysis using the eastern blotting determined a new marker compound causing an adverse effect.
Fujii S, Morinaga O, Uto T, Nomura S, Shoyama Y. Development of a monoclonal antibody-based immunochemical assay for liquiritin and its application to the quality control of licorice products. J Agric Food Chem. 2014;62(15):3377–83. https://doi.org/10.1021/jf404731z.
Tanaka H, Fukuda N, Shoyama Y. Formation of monoclonal antibody against a major ginseng component, ginsenoside Rb1 and its characterization. Cytotechnology. 1999;29(2):115–20. https://doi.org/10.1023/A:1008068718392.
Shoyama Y, Tanaka H. Quantitative analysis of ginsenosides Rb1, Rg1, and Re in American ginseng berry and flower samples by ELISA using monoclonal antibodies. J Nat Med. 2009;63(3):360–3. https://doi.org/10.1007/s11418-009-0332-x.
Sritularak B, Morinaga O, Yuan CS, Shoyama Y, Tanaka H. Quantitative analysis of ginsenosides Rb1, Rg1, and Re in American ginseng berry and flower samples by ELISA using monoclonal antibodies. J Nat Med. 2009;63(3):360–3. https://doi.org/10.1007/s11418-009-0332-x.
Chao Z, Shoyama Y, Tanaka H. Pharmacokinetic study of ginsenosides Rb1 and Rg1 in rat by ELISA using anti-ginsenosides Rb1 and Rg1 monoclonal antibodies. Am J Chin Med. 2006;34(6):1069–81. https://doi.org/10.1142/S0192415X06004533.
Ma LL, Chao Z, Tanaka H, Shoyama Y. Immunodetection of ginsenoside Rb1 in rat serum. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27(12):1915–7.
Tanaka H, Fukuda N, Yahara S, Isoda S, Yuan CS, Shoyama Y. Isolation of ginsenoside Rb1 from Kalopanax pictus by eastern blotting using anti-ginsenoside Rb1 monoclonal antibody. Phytother Res. 2005;19(3):255–8. https://doi.org/10.1002/ptr.1675.
Tung NH, Shoyama Y. Eastern blotting analysis and isolation of two new dammarane-type saponins from American ginseng. Chem Pharm Bull (Tokyo). 2012;60(10):1329–33. https://doi.org/10.1248/cpb.c12-00486.
Fukuda N, Tanaka H, Shoyama Y. Double staining of ginsenosides by Western blotting using anti-ginsenoside Rb1 and Rg1 monoclonal antibodies. Biol Pharm Bull. 2001;24(10):1157–60. https://doi.org/10.1248/bpb.24.1157.
Tanaka H, Fukuda N, Shoyama Y. Eastern blotting and immunoaffinity concentration using monoclonal antibody for ginseng saponins in the field of traditional chinese medicines. J Agric Food Chem. 2007;55(10):3783–7. https://doi.org/10.1021/jf063457m.
Yokota S, Onohara Y, Shoyama Y. Immunofluorescence and immunoelectron microscopic localization of medicinal substance, Rb1, in several plant parts of Panax ginseng. Curr Drug Discov Technol. 2011;8(1):51–9. https://doi.org/10.2174/157016311794519938.
Fukuda N, Tanaka H, Shoyama Y. Formation of monoclonal antibody against a major ginseng component, ginsenoside Rg1 and its characterization. Monoclonal antibody for a ginseng saponin. Cytotechnology. 2000;34(3):197–204. https://doi.org/10.1023/A:1008162703957.
Morinaga O, Tanaka H, Shoyama Y. Detection and quantification of ginsenoside Re in ginseng samples by a chromatographic immunostaining method using monoclonal antibody against ginsenoside Re. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;830(1):100–4. https://doi.org/10.1016/j.jchromb.2005.10.040.
Pongkitwitoon B, Sakamoto S, Morinaga O, Juengwatanatrakul T, Shoyama Y, Tanaka H, et al. Single-chain variable fragment antibody against ginsenoside Re as an effective tool for the determination of ginsenosides in various ginsengs. J Nat Med. 2011;65(1):24–30. https://doi.org/10.1007/s11418-010-0446-1.
Sakamoto S, Taura F, Pongkitwitoon B, Putalun W, Tsuchihashi R, Kinjo J, et al. Development of sensitivity-improved fluorescence-linked immunosorbent assay using a fluorescent single-domain antibody against the bioactive naphthoquinone, plumbagin. Anal Bioanal Chem. 2010;396(8):2955–63. https://doi.org/10.1007/s00216-010-3535-9.
Morinaga O, Uto T, Yuan CS, Tanaka H, Shoyama Y. Evaluation of a new eastern blotting technique for the analysis of ginsenoside Re in American ginseng berry pulp extracts. Fitoterapia. 2010;81(4):284–8. https://doi.org/10.1016/j.fitote.2009.10.005.
Fukuda N, Tanaka H, Shoyama Y. Isolation of the pharmacologically active saponin ginsenoside Rb1 from ginseng by immunoaffinity column chromatography. J Nat Prod. 2000;63(2):283–5. https://doi.org/10.1021/np990356s.
• Pongkitwitoon B, Sakamoto S, Nagamitsu R, Putalun W, Tanaka H, Morimoto S. A monoclonal antibody-based enzyme-linked immunosorbent assay for determination of homoharringtonine. Planta Med. 2018;84(14):1038–44. https://doi.org/10.1055/a-0578-8689This article is one example of ELISA using MAbs against natural compounds.
• Sakamoto S, Yusakul G, Nuntawong P, Kitisripanya T, Putalun W, Miyamoto T, et al. Development of an indirect competitive immunochromatographic strip test for rapid detection and determination of anticancer drug, harringtonine. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1048:150–4. https://doi.org/10.1016/j.jchromb.2017.01.032This article is one example of immunochromatographic strip assay using MAbs against natural compounds.
•• Sakamoto S, Miyamoto T, Usui K, Tanaka H, Morimoto S. Sodium-periodate-mediated harringtonine derivatives and their antiproliferative activity against HL-60 acute leukemia cells. J Nat Prod. 2018;81(1):34–40. https://doi.org/10.1021/acs.jnatprod.7b00541This article demonstrates the analysis of cellular uptake of natural compounds using ELISA.
Kido K, Morinaga O, Shoyama Y, Tanaka H. Quick analysis of baicalin in Scutellariae Radix by enzyme-linked immunosorbent assay using a monoclonal antibody. Talanta. 2008;77(1):346–50. https://doi.org/10.1016/j.talanta.2008.06.034.
Uto T. Functional analysis of bioactive natural compounds using monoclonal antibodies against natural compounds. Yakugaku Zasshi. 2014;134(10):1061–7. https://doi.org/10.1248/yakushi.14-00178.
Tian M, Tanaka H, Shang MY, Karashima S, Chao Z, Wang X, et al. Production, characterization of a monoclonal antibody against aristolochic acid-II and development of its assay system. Am J Chin Med. 2008;36(2):425–36. https://doi.org/10.1142/S0192415X08005874.
Li XW, Morinaga O, Tian M, Uto T, Yu J, Shang MY, et al. Development of an eastern blotting technique for the visual detection of aristolochic acids in Aristolochia and Asarum species by using a monoclonal antibody against aristolochic acids I and II. Phytochem Anal. 2013;24(6):645–53. https://doi.org/10.1002/pca.2448.
Li XW, Yokota S, Wang D, Wang X, Shoyama Y, Cai SQ. Localization of aristolochic acid in mouse kidney tissues by immunohistochemistry using an anti-AA-I and AA-II monoclonal antibody. Am J Chin Med. 2014;42(6):1453–69. https://doi.org/10.1142/S0192415X14500918.
Wang D, Li XW, Wang X, Tan HR, Jia Y, Yang L, et al. Alpha-actinin-4 is a possible target protein for aristolochic acid I in human kidney cells in vitro. Am J Chin Med. 2016;44(2):291–304. https://doi.org/10.1142/S0192415X16500178.
Ishiyama M, Shoyama Y, Murakami H, Shinohara H. Production of monoclonal antibodies and development of an ELISA for solamargine. Cytotechnology. 1995;18(3):153–8. https://doi.org/10.1007/BF00767762.
Putalun W, Tanaka H, Yahara S, Lhieochaiphan S, Shoyama Y. Survey of solasodine-type glycoalkaloids by western blotting and ELISA using anti-solamargine monoclonal antibody. Biol Pharm Bull. 2000;23(1):72–5. https://doi.org/10.1248/bpb.23.72.
Putalun W, Tanaka H, Shoyama Y. Rapid separation of solasodine glycosides by an immunoaffinity column using anti-solamargine monoclonal antibody. Cytotechnology. 1999;31(1–2):153–8. https://doi.org/10.1023/A:1008032524419.
Putalun W, Taura F, Qing W, Matsushita H, Tanaka H, Shoyama Y. Anti-solasodine glycoside single-chain Fv antibody stimulates biosynthesis of solasodine glycoside in plants. Plant Cell Rep. 2003;22(5):344–9. https://doi.org/10.1007/s00299-003-0689-3.
Morinaga O, Uto T, Sakamoto S, Tanaka H, Shoyama Y. Enzyme-linked immunosorbent assay for total sennosides using anti-sennoside A and anti-sennoside B monoclonal antibodies. Fitoterapia. 2009;80(1):28–31. https://doi.org/10.1016/j.fitote.2008.09.004.
Morinaga O, Uto T, Sakamoto S, Putalun W, Lhieochaiphant S, Tanaka H, et al. Development of eastern blotting technique for sennoside A and sennoside B using anti-sennoside A and anti-sennoside B monoclonal antibodies. Phytochem Anal. 2009;20(2):154–8. https://doi.org/10.1002/pca.1111.
Zhu SH, Shimokawa S, Tanaka H, Shoyama Y. Development of an assay system for saikosaponin A using anti-saikosaponin A monoclonal antibodies. Biol Pharm Bull. 2004;27(1):66–71. https://doi.org/10.1248/bpb.27.66.
Zhu S, Shimokawa S, Shoyama Y, Tanaka H. A novel analytical ELISA-based methodology for pharmacologically active saikosaponins. Fitoterapia. 2006;77(2):100–8. https://doi.org/10.1016/j.fitote.2005.11.005.
Chao Z, Cui Q, Tian E, Zeng W, Cai X, Li X, et al. Ultrasensitive time-resolved fluoroimmunoassay for saikosaponin A in Chaihu (Bupleuri Radix). PLoS One. 2016;11(3):e0151032. Published 2016 Mar 11. https://doi.org/10.1371/journal.pone.0151032.
Lu Z, Morinaga O, Tanaka H, Shoyama Y. A quantitative ELISA using monoclonal antibody to survey paeoniflorin and albiflorin in crude drugs and traditional Chinese herbal medicines. Biol Pharm Bull. 2003;26(6):862–6. https://doi.org/10.1248/bpb.26.862.
Lu Z, Masaki T, Shoyama Y, Tanaka H. Construction and expression of a single chain Fv fragment against pharmacologically active paeoniflorin in Escherichia coli, and its potential use in an enzyme-linked immunosorbent assay. Planta Med. 2006;72(2):151–5. https://doi.org/10.1055/s-2005-873188.
Morinaga O, Lu Z, Lin L, Uto T, Sangmalee S, Putalun W, et al. Detection of paeoniflorin and albiflorin by immunostaining technique using anti-paeoniflorin monoclonal antibody. Phytochem Anal. 2013;24(2):124–8. https://doi.org/10.1002/pca.2389.
Kim JS, Tanaka H, Shoyama Y. Immunoquantitative analysis for berberine and its related compounds using monoclonal antibodies in herbal medicines. Analyst. 2004;129(1):87–91. https://doi.org/10.1039/b311304c.
Kim JS, Masaki T, Sirikantaramas S, Shoyama Y, Tanaka H. Activation of a refolded, berberine-specific, single-chain Fv fragment by addition of free berberine. Biotechnol Lett. 2006;28(13):999–1006. https://doi.org/10.1007/s10529-006-9033-7.
Chao Z, Tan M, Paudel MK, Sakamoto S, Ma L, Sasaki-Tabata K, et al. Development of an indirect competitive enzyme-linked immunosorbent assay (icELISA) using highly specific monoclonal antibody against paclitaxel. J Nat Med. 2013;67(3):512–8. https://doi.org/10.1007/s11418-012-0708-1.
• Cui Q, Tanaka H, Shoyama Y, Ye HT, Li F, Tian EW, et al. Development of a competitive time-resolved fluoroimmunoassay for paclitaxel. Phytochem Anal. 2018;29(3):284–9. https://doi.org/10.1002/pca.2741This article is one example of application using MAbs against natural compounds.
• Yusakul G, Sakamoto S, Tanaka H, Morimoto S. Improvement of heavy and light chain assembly by modification of heavy chain constant region 1 (CH1): application for the construction of an anti-paclitaxel fragment antigen-binding (Fab) antibody. J Biotechnol. 2018;288:41–7. https://doi.org/10.1016/j.jbiotec.2018.10.009This article is one example of application using MAbs against natural compounds.
Sakamoto S, Putalun W, Tsuchihashi R, Morimoto S, Kinjo J, Tanaka H. Development of an enzyme-linked immunosorbent assay (ELISA) using highly-specific monoclonal antibodies against plumbagin. Anal Chim Acta. 2008;607(1):100–5. https://doi.org/10.1016/j.aca.2007.11.021.
Sakamoto S, Taura F, Putalun W, Pongkitwitoon B, Tsuchihashi R, Morimoto S, et al. Construction and expression of specificity-improved single-chain variable fragments against the bioactive naphthoquinone, plumbagin. Biol Pharm Bull. 2009;32(3):434–9. https://doi.org/10.1248/bpb.32.434.
Sakamoto S, Putalun W, Pongkitwitoon B, Juengwatanatrakul T, Shoyama Y, Tanaka H, et al. Modulation of plumbagin production in Plumbago zeylanica using a single-chain variable fragment antibody against plumbagin. Plant Cell Rep. 2012;31(1):103–10. https://doi.org/10.1007/s00299-011-1143-6.
• Yusakul G, Togita R, Minami K, Chanpokapaiboon K, Juengwatanatrakul T, Putalun W, et al. An indirect competitive enzyme-linked immunosorbent assay toward the standardization of Pueraria candollei based on its unique isoflavonoid, kwakhurin. Fitoterapia. 2019;133:23–8. https://doi.org/10.1016/j.fitote.2018.12.010This article is one example of ELISA using MAbs against natural compounds.
• Chanpokapaiboon K, Khoonrit P, Yusakul G, Juengwatanatrakul T, Putalun W, Tanaka H, et al. A recombinant fab antibody against kwakhurin as a tool for sensitive indirect competitive ELISA. Curr Pharm Biotechnol. 2018;19(14):1170–6. https://doi.org/10.2174/1389201020666181226105223This article is one example of application using MAbs against natural compounds.
Singh KV, Kaur J, Varshney GC, Raje M, Suri CR. Synthesis and characterization of hapten-protein conjugates for antibody production against small molecules. Bioconjug Chem. 2004;15(1):168–73. https://doi.org/10.1021/bc034158v.
Goodrow MH, Harrison RO, Hammock BD. Hapten synthesis, antibody development, and competitive inhibition enzyme immunoassay for s-triazine herbicides. J Agric Food Chem. 1990;38(4):990–6. https://doi.org/10.1021/jf00094a016.
Szurdoki F, Székács A, Le HM, Hammock BD. Synthesis of haptens and protein conjugates for the development of immunoassays for the insect growth regulator fenoxycarb. J Agric Food Chem. 2002;50(1):29–40. https://doi.org/10.1021/jf0107050.
Shoyama Y, Fukada T, Tanaka T, Kusai A, Nojima K. Direct determination of opium alkaloid-bovine serum albumin conjugate by matrix-assisted laser desorption/ionization mass spectrometry. Biol Pharm Bull. 1993;16(10):1051–3. https://doi.org/10.1248/bpb.16.1051.
Goto Y, Shima Y, Morimoto S, Shoyama Y, Murakami H, Kusai A, et al. Determination of tetrahydrocannabinolic acid–carrier protein conjugate by matrix-assisted laser desorption/ionization mass spectrometry and antibody formation. Org Mass Spectrom. 1994;29:668–71. https://doi.org/10.1002/oms.1210291115.
Pérard-Viret J, Quteishat L, Alsalim R, Royer J, Dumas F. Cephalotaxus alkaloids. Alkaloids Chem Biol. 2017;78:205–352. https://doi.org/10.1016/bs.alkal.2017.07.001.
Powell RG, Weisleder D, Smith CR Jr, Rohwedder WK. Structures of harringtonine, isoharringtonine, and homoharringtonine. Tetrahedron Lett. 1970;11:815–8. https://doi.org/10.1016/s0040-4039(01)97839-6.
Powell RG, Weisleder D, Smith CR Jr, Wolff IA. Structure of cephalotaxine and related alkaloids. Tetrahedron Lett. 1969;10:4081–4. https://doi.org/10.1016/S0040-4039(01)88620-2.
Lee IS, Kang KS, Kim SY. Panax ginseng pharmacopuncture: current status of the research and future challenges. Biomolecules. 2019;10(1):33. https://doi.org/10.3390/biom10010033.
Yu SE, Mwesige B, Yi YS, Yoo BC. Ginsenosides: the need to move forward from bench to clinical trials. J Ginseng Res. 2019;43(3):361–7. https://doi.org/10.1016/j.jgr.2018.09.001.
Kao TC, Wu CH, Yen GC. Bioactivity and potential health benefits of licorice. J Agric Food Chem. 2014;62(3):542–53. https://doi.org/10.1021/jf404939f.
Conn JW, Rovner DR, Cohen EL. Licorice-induced pseudoaldosteronism. Hypertension, hypokalemia, aldosteronopenia, and suppressed plasma renin activity. JAMA. 1968;205(7):492–6. https://doi.org/10.1001/jama.205.7.492.
Akao T, Hayashi T, Kobashi K, Kanaoka M, Kato H, Kobayashi M, et al. Intestinal bacterial hydrolysis is indispensable to absorption of 18 beta-glycyrrhetic acid after oral administration of glycyrrhizin in rats. J Pharm Pharmacol. 1994;46(2):135–7. https://doi.org/10.1111/j.2042-7158.1994.tb03756.x.
Monder C, Stewart PM, Lakshmi V, Valentino R, Burt D, Edwards CR. Licorice inhibits corticosteroid 11 beta-dehydrogenase of rat kidney and liver: in vivo and in vitro studies. Endocrinology. 1989;125(2):1046–53. https://doi.org/10.1210/endo-125-2-1046.
Kato H, Kanaoka M, Yano S, Kobayashi M. 3-Monoglucuronyl-glycyrrhetinic acid is a major metabolite that causes licorice-induced pseudoaldosteronism. J Clin Endocrinol Metab. 1995;80(6):1929–33. https://doi.org/10.1210/jcem.80.6.7775643.
Akao T. Distribution of enzymes involved in the metabolism of glycyrrhizin in various organs of rat. Biol Pharm Bull. 1998;21(10):1036–44. https://doi.org/10.1248/bpb.21.1036.
Ohtake N, Kido A, Kubota K, Tsuchiya N, Morita T, Kase Y, et al. A possible involvement of 3-monoglucuronyl-glycyrrhetinic acid, a metabolite of glycyrrhizin (GL), in GL-induced pseudoaldosteronism. Life Sci. 2007;80(17):1545–52. https://doi.org/10.1016/j.lfs.2007.01.033.
Asl MN, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res. 2008;22(6):709–24. https://doi.org/10.1002/ptr.2362.
Wang ZY, Nixon DW. Licorice and cancer. Nutr Cancer. 2001;39(1):1–11. https://doi.org/10.1207/S15327914nc391_1.
Anger EE, Yu F, Li J. Aristolochic acid-induced nephrotoxicity: molecular mechanisms and potential protective approaches. Int J Mol Sci. 2020;21(3):1157. Published 2020 Feb 10. https://doi.org/10.3390/ijms21031157.
Funding
This work was financially supported by JSPS KAKENHI Grant Numbers 19K07145, 25871011, and 15K16225.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Food Factors: Molecular Targets, Mechanisms, Pharmacology and In Vivo Efficacy
Rights and permissions
About this article
Cite this article
Uto, T., Fujii, S., Sakamoto, S. et al. Applications of Monoclonal Antibodies Against Natural Compounds for Functional Analysis of Crude Drugs. Curr Pharmacol Rep 6, 192–201 (2020). https://doi.org/10.1007/s40495-020-00224-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-020-00224-7