Abstract
Background and Objectives
Ezetimibe/simvastatin combination tablet has been approved for the treatment of high low-density lipoprotein cholesterol (LDL-C) levels in patients with primary hypercholesterolaemia or mixed hypercholesterolaemia as adjunctive therapy to diet, when diet alone is insufficient in lowering cholesterol. The aims of this study were to assess the pharmacokinetics and safety of an ezetimibe/simvastatin combination tablet after oral single-dose administration in healthy Chinese subjects including sex-related differences in pharmacokinetics.
Methods
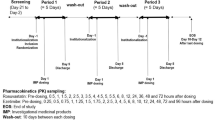
This was an open-label, single-dose study. Twelve healthy subjects (six males and six females) received a single dose of an ezetimibe/simvastatin combination tablet (ezetimibe 10 mg and simvastatin 40 mg). The pharmacokinetic parameters for ezetimibe and simvastatin were assessed by determining total ezetimibe, free ezetimibe, simvastatin and simvastatin acid concentrations using a validated liquid chromatography–tandem mass spectrometry method. Safety was evaluated by monitoring adverse events, laboratory assays, vital signs, physical examinations and 12-lead electrocardiograms.
Results
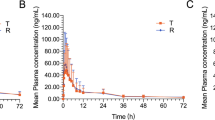
The pharmacokinetic parameters (mean ± SD) for total ezetimibe and free ezetimibe following a single dose were: maximum plasma drug concentration (Cmax) 81.56 ± 26.62 and 9.40 ± 6.17 ng/mL; time to reach Cmax (tmax) 0.93 ± 0.30 and 1.25 ± 1.27 h; elimination half-life (t½) 24.32 ± 13.27 and 18.90 ± 9.66 h, and mean area under the plasma concentration–time curve from time zero to the time of the last measurable concentration (AUClast) 579.06 ± 241.45 and 126.01 ± 69.01 ng·h/mL, respectively. The pharmacokinetic parameters (mean ± SD) for simvastatin and simvastatin acid following a single dose were: Cmax 11.92 ± 5.50 and 3.37 ± 1.78 ng/mL, tmax 0.98 ± 0.28 and 3.73 ± 1.68 h, t½ 4.19 ± 1.81 and 7.65 ± 7.96 h, and mean AUClast 33.63 ± 20.41 and 32.50 ± 18.79 ng·h/mL. Higher AUClast and AUC from time zero to infinity (AUC∞), and lower apparent total body clearance of drug from plasma after oral administration (CL/F) for total ezetimibe and free ezetimibe were observed in female subjects compared with those in male subjects. There were no differences between the pharmacokinetic parameters of simvastatin and simvastatin acid for female and male subjects in the study.
Conclusion
Ezetimibe/simvastatin combination tablet has a generally favourable safety and tolerability profile in healthy Chinese subjects. A statistically significant difference with regard to sex in the pharmacokinetics of ezetimibe was observed. Sex had no effect on the pharmacokinetics of simvastatin and simvastatin acid.

Similar content being viewed by others
References
Isaacsohn J, Hunninghake D, Schrott H, et al. Effects of simvastatin, an HMG-CoA reductase inhibitor, in patients with hypertriglyceridemia. Clinl Cardiol. 2003;26(1):18–24.
Waters DD, Brotons C, Chiang CW, et al. Lipid treatment assessment project 2: a multinational survey to evaluate the proportion of patients achieving low-density lipoprotein cholesterol goals. Circulation. 2009;120(1):28–34.
Gotto AM. Statins, cardiovascular disease, and drug safety. Am J Cardiol. 2006;97(suppl):S3–5.
Altmann SW, Davis HR, Zhu LJ, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303(5661):1201–4.
Jeu LA, Cheng WM. Pharmacology and therapeutics of ezetimibe (SCH58235), a cholesterol-absorption inhibitor. Clin Ther. 2003;25(9):2352–87.
Bays HE, Moore PB, Drehobl MA, et al. Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: pooled analysis of two phase II studies. Clin Ther. 2001;23(8):1209–30.
Davis HR. Ezetimibe: first in a new class of cholesterol absorption inhibitors. Int Congr. 2004;1262(5):243–6.
Dujovne CA, Ettiinger MP, McNeer JF, et al. Efficacy and safety of a potent new selective cholesterol absorption, ezetimibe, in patients with primary hypercholesterolemia. Am J Cardiol. 2002;90(10):1092–7.
Bays HE, Ose L, Fraser N, et al. A multicenter, randomized, double-blind, placebo-controlled, factorial design study to evaluate the lipid-altering efficacy and safety profile of the Ezetimibe/simvastatin tablet compared with ezetimibe and simvastatin monotherapy in patients with primary hypercholesterolemia. Clin Ther. 2004;26(11):1758–73.
Anouk G, Cynthia CJ, Maud NV, et al. Efficacy and safety of coadministration of ezetimibe and simvastatin in adolescents with heterozygous familial hypercholesterolemia. J Am Coll Cardiol. 2008;52(17):1421–9.
Nicola A, Alberico LC, Christie MB, et al. Effect of ezetimibe/simvastatin versus atorvastatin or rosuvastatin on modifying lipid profiles in patients with diabetes, metabolic syndrome, or neither: results of two subgroup analyses. J Clin Lipidol. 2008;2(2):91–105.
John RG, Ronald BG, Theodore M, et al. Lipoprotein and apolipoprotein ratios in the VYTAL trial of ezetimibe/simvastatin compared with atorvastatin in type 2 diabetes. J Clin Lipidol. 2008;2(1):19–24.
Davidson MH, McGarry T, Bettis R, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40(12):2125–34.
Pearson T, Ballantyne C, Sisk C, et al. Comparison of effects of ezetimibe/simvastatin versus simvastatin versus atorvastatin in reducing C-reactive protein and low-density lipoprotein cholesterol levels. Am J Cardiol. 2007;99(12):1706–13.
Ballantyne CM, Blazing MA, King TR, et al. Efficacy and safety of ezetimibe co-administered with simvastatin compared with atorvastatin in adults with hypercholesterolemia. Am J Cardiol. 2004;93(12):1487–94.
Robinson JG, Ballantyne CM, Grundy SM, et al. Lipid-altering efficacy and safety of ezetimibe/simvastatin versus atorvastatin in patients with hypercholesterolemia and the metabolic syndrome (from the VYMET Study). Am J Cardiol. 2009;103(12):1694–702.
Ballantyne CM, Abate N, Yuan Z, et al. Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: The Vytorin Versus atorvastatin (VYVA) Study. Am Heart J. 2005;149(3):464–73.
Kashani A, Sallam T, Bheemreddy S, et al. Review of side-effect profile of combination ezetimibe and statin therapy in randomized clinical trials. Am J Cardiol. 2008;101(11):1606–13.
Migoya EM, Bergman A, Hreniuk D, et al. Bioequivalence of an ezetimibe/simvastatin combination tablet and coadministration of ezetimibe and simvastatin as separate tablets in healthy subjects. Int J Clin Pharmacol Ther. 2006;44(2):83–92.
Ballantyne CM. Ezetimibe: efficacy and safety in clinical trials. Eur Heart J 2002;4 (suppl J):J9–J18.
Kosoglou T, Meyer I, Veltri EP, et al. Pharmacodynamic interaction between the new selective cholesterol absorption inhibitor ezetimibe and simvastatin. Br J Clin Pharmacol. 2002;54(3):309–19.
Zetia® (ezetimibe) drug monograph. http://www.pharmacy.cuhk.edu.hk/ampoule/en/monograph/ezetimibe.pdf. Accessed 2010 Nov 1.
Kosoglou T, Statkevich P, Johnson-Levonas AO, et al. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44(5):467–94.
ZOCOR® (Simvastatin) tablets. Merck & Co., INC. Whitehouse Station, NJ08889, USA.
European Medicines Agency (EMEA) Guideline for Good Clinical Practice ICH Topic E 6 (R1). July 2002, CPMP/ICH/135/95. http://www.emea.europa.eu/pdfs/human/ich/013595en.pdf. Accessed 2009 Oct 28.
World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. http://www.wma.net/en/30publications/10polocies/b3/index.html. Accessed 2009 Oct 28.
Li S, Liu G, Jia J, Li X, Yu C, et al. Liquid chromatography-negative ion electrospray tandem mass spectrometry method for the quantification of ezetimibe in human plasma. J Pharm Biomed Anal. 2006;40(4):987–92.
Barrett B, Huclova J, Dohalsky VB, et al. Validated HPLC-MS/MS method for simultaneous determination of simvastatin and simvastatin hydroxyl acid in human plasma. J Pharm Biomed Anal. 2006;41(2):517–26.
Bennett SK, Huttner RP, Lipka L, et al. Efficacy and safety of ezetimibe coadministered with statins in male and female patients. Obstet Gynecol. 2004;109:966–71. (Abstract).
Zetia [package insert]. North Wales (PA): Merck/Schering-Plough Pharmaceuticals, 2005.
Najib NM, Idkaidek N, Adel A, et al. Pharmacokinetics and bioequivalence evaluation of two simvastatin 40 mg tablets (Simvast & Zocor) in healthy human volunteers. Biopharm Drug Dispos. 2003;24(5):183–9.
Acknowledgements
This study was sponsored by Schering-Plough (China) Ltd, which was involved in the design of the study and in reviewing the manuscript. The authors thank Chen Yu, Gang-Yi Liu and Jing-Ying Jia, Shanghai Xuhui Central Hospital, China, for assistance with the blood sample analysis, and Joanne E. Tomassini, Merck, North Wales, PA, USA, for editorial assistance. The authors have indicated that they have no other conflicts of interest with regard to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chu, NN., Chen, WL., Xu, HR. et al. Pharmacokinetics and Safety of Ezetimibe/Simvastatin Combination Tablet. Clin Drug Investig 32, 791–798 (2012). https://doi.org/10.1007/s40261-012-0013-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-012-0013-5