Abstract
Background
Ezetimibe is a new class of antihyperlipidemic agent indicated for the prevention of atherosclerosis disease and for the treatment of hypercholesterolemia. Information on the pharmacokinetic profiles of ezetimibe tablet in healthy Chinese volunteers are lacking, and regulatory requirements necessitate a bioequivalence study of ezetimibe tablet versus Ezetrol® in China.
Methods
A single-dose randomized, open-label, two-group, two-period crossover study was conducted in 59 healthy Chinese volunteers under fasting or fed conditions to assess the bioequivalence between two preparations. Eligible participants were randomly divided into fasted and fed groups. Blood samples were collected at specified time intervals, and the plasma concentrations of ezetimibe and ezetimibe glucuronide were determined by a validated liquid chromatography-tandem mass spectrometry (LC–MS/MS) method. PK and bioavailability parameters were estimated via non-compartmental methods. Adverse events were also recorded.
Results
Fifty-nine healthy volunteers were enrolled in the study. The main pharmacokinetic parameters of total ezetimibe in the plasma of the ezetimibe tablet (10 mg) and the Ezetrol® (10 mg) after a single fasting administration: Cmax were (65.73 ± 47.14), (71.32 ± 51.98) ng·mL− 1; Tmax were 1.75, 1.25 h; T½ were (17.09 ± 13.22), (17.35 ± 12.14) h; AUC0-t were (643.34 ± 400.77), (668.49 ± 439.57) h·ng·mL− 1; AUC0-∞ were (706.36 ± 410.92), (734.23 ± 468.26) h·ng·mL− 1. The main pharmacokinetic parameters of total ezetimibe in plasma of ezetimibe tablet (10 mg) and Ezetrol® (10 mg) after a fed administration: Cmax were (83.38 ± 38.95), (84.74 ± 34.62) ng·mL− 1; Tmax were 2.50, 2.50 h; T½ were (22.56 ± 12.68), (19.80 ± 15.59) h; AUC0-t were (494.21 ± 208.65), (536.69 ± 209.11) h·ng·mL− 1; AUC0-∞ were (573.74 ± 252.74), (604.75 ± 247.13) h·ng·mL− 1. The main pharmacokinetic parameters Cmax, AUC0-t, and AUC0-∞ of the two drugs were analyzed by variance analysis after logarithmic transformation. The total ezetimibe under fasting state with 90% confidence intervals (CIs) were 85.29 ~ 97.19, 90.41% ~ 104.38%, and 90.81 ~ 106.05%; total ezetimibe in fed state were 86.36% ~ 109.17, 84.96% ~ 96.40, and 85.32% ~ 101.0%. The 90% CIs of the ratio of geometric means (GMRs) of Cmax, AUC0-t, and AUC0-∞ of Ezetrol® and ezetimibe tablet both fasting and fed conditions fell within the conventional bioequivalence criteria of 0.80–1.25. Both Cmax and AUC met the predetermined criteria for assuming bioequivalence. No severe adverse events were observed.
Conclusions
The test ezetimibe tablet and Ezetrol® were determined to be bioequivalent under both fasting and fed conditions in Chinese people.
Trial registration
Clinicaltrials, NCT05681247 (retrospectively registered in 11/01/ 2023).
Similar content being viewed by others
Background
Ezetimibe (Ezetrol), as a non-statin lipid-lowering approach, was approved by US Food and Drug Administration (FDA) in patients with hypercholesterolemia (https://en.wikipedia.org/wiki/Ezetimibe, (https://pubchem.ncbi.nlm.nih.gov/compound/Ezetimibe#section=Top, https://www.drugbank.ca/drugs/DB00973, [1, 2]) Ezetimibe, administered in combination with a 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor (statin), is indicated as adjunctive therapy to diet for the reduction of elevated total-C, LDL-C, Apo B, and non-HDL-C in patients with primary (heterozygous familial and non-familial) hyperlipidemia ([3,4,5], https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=A773B0B2-D31C-4FF4-B9E8-1EB2D3A4D62A) Ezetimibe reduces blood cholesterol by inhibiting the absorption of cholesterol by the small intestine. It takes effect through acting at the brush border of the small intestine and inhibiting the absorption of cholesterol, which leading to a decrease of hepatic cholesterol stores and an increase in clearance of blood cholesterol. After oral administration, ezetimibe is rapidly and extensively metabolized in the intestinal wall and the liver to a corresponding phenol glucuronide. This glucuronide is reexcreted in the bile back to its active site [6].
In this study, it is meaningful to investigate the pharmacokinetic characteristics and bioequivalence of single-dose ezetimibe tablets and Ezetrol® in Chinese healthy subjects under fasting and fed conditions.
Methods
Ethics approval
The trial was performed abiding by the Declaration of Helsinki [7], Good clinical practice (GCP) [8] and the guidelines of China National Medical Products Administration (NMPA). Relevant documents were all approved independently by the Medical Ethics Committee of the Affiliated Hospital of Qingdao University (Ethics approval No. QYFYEC 2017–026-01). Written informed consent was obtained from all subjects before their participation in the study. All protocol violations have been reported to the Medical Ethics Committee.
Subjects
Chinese males and females aged 18 and above with a body mass index in the range of 18.0–26.0 kg/m2 (including the boundary value) were eligible for inclusion. The body weight of males and females was at least 50 kg and 45 kg, respectively. Serum total cholesterol was between 2.9 and 5.0 mmol/L (not including the boundary value). All volunteers were healthy, as confirmed by medical history, physical examination, vital sign measurements, 12-lead ECG and laboratory safety tests including hematology and urinalysis. The exclusion criteria included as follows: patients with medical history of cardiovascular, digestive, respiratory, nervous or hematological diseases, abnormal vital signs, allergic to ezetimibe or its excipients, lactating or pregnant women,drug or alcohol abuse, smoking≥5 cigarettes per day, donation (≥300 ml) or enrollment in other clinical trials within 3 months, any use of prescription drugs or vitamins or caffeine/xanthine-rich beverages 48 h prior to taking medication.
Study design
This was a randomized, single center, open-label, single-dose, two-period crossover phase I clinical trial designed to enroll healthy volunteers with the aim of comparing PK parameters of Ezetrol® (10 mg, batch no.5 EZPA42002, from MSD Pharma (Singapore) Pte. Ltd.) to those of ezetimibe tablet (10 mg, batch no. 170301, from Chongqing shenghuaxi Pharmaceutical Co., Ltd.). All study drugs were provided by the sponsor at no cost.
Randomization was planned to generate two subject groups (fasting conditions and fed conditions) with administration of the products in two stages, separated by a 14-day washout period. Each eligible subject will be given a randomization number from small to large according to the screening number.
The qualified volunteers were hospitalized in the phase I clinical research center on the day before dosing and placed on a uniform diet during hospitalization. The fasting group were fasted for overnight (10 h) before administration. The fed group was requested to take a high-fat breakfast (total energy 1000 cal, 65% fat, 15% protein, 20% carbohydrate) within 0.5 h before administration. The management provided standard lunch and dinner for participants at 4 h and 10 h after dosing, separately. They then returned to the facility for regular blood sampling up to a total duration of 14 days. Blood samples (4 ml) were collected in precooled vacuum tubes containing sodium citrate anticoagulant in each stage (pre-administration and at 0.16, 0.3, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, 24, 36, 48, 72 h after administration).
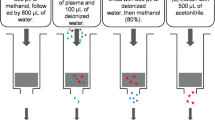
The plasma samples were centrifuged at 4000 g for 10 min at 4 °C and future stored at − 80 °C. Bio-analysis experiment was analyzed by LC–MS/MS methods at Chongqing shengshifuma Technology Co., Ltd, [9]. Accuracy expressed as bias ranged from − 0.7 to 1.8% and 0.7 to 3.5% for ezetimibe and ezetimibe glucuronide. The lower limit of ezetimibe and ezetimibe glucuronide were 0.15 ng/mL and 1.5 ng/mL respectively.
Safety and tolerability evaluations
The tolerability and safety of ezetimibe tablet were evaluated based on adverse-event reports, physical examinations, vital signs (body temperature, blood pressure and heart rate), 12-lead ECG and clinical laboratory tests (serum chemistry, hematology and urinalysis). Vital signs, were measured in each stage (screening, pre-administration and at 1, 4, 12, 24, 48, 72 h after administration). Routine laboratory tests and 12-lead ECG were conducted at screening and before removal from the study (18 days). Adverse events (AEs) were monitored throughout the study and were graded in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
Pharmacokinetic analysis
Pharmacokinetic parameters of ezetimibe and ezetimibe glucuronide were calculated with DAS 3.3.1 software by noncompartmental analysis method. The primary PK parameters were the maximum plasma concentration (Cmax), the area under the plasma concentration-time curve from 0 to the last measured time point (AUC0-t), and the area under the plasma concentration-time curve from 0 to infinity (AUC0-∞). The secondary PK parameters were the observed time to Cmax (Tmax) and the apparent terminal half-life (T½). Cmax and Tmax were obtained from the date and AUC0-t was calculated using the linear and logarithmic trapezoidal rule. AUC0-∞ was calculated as the sum of AUC0-t and Clast/λz (Clast is the last measurable concentration and λz is the slope of linear regression after logarithmic conversion at the end of the concentration–time curve). T½ was calculated to be ln2/λ [10].
Statistical analysis
Statistical analysis was performed by SAS 9.4 (SAS Institute Inc. Cary, NC, USA). Demographic characteristics, safety parameters and PK data were summarized using descriptive statistics,.Statistical data were presented as mean ± standard deviation (SD) The differences between groups were determined by two one-sided tests [10]. The probability value less than 0.05 was considered statistically significant.
Analysis of variance (ANOVA) was performed on the logarithmically transformed Cmax, AUC0-t and AUC0-∞. A mixed-effect model that included treatment group, period and formulation as fixed effects and subject within sequence as random effect was used for all comparisons. The geometric mean ratios (GMRs) ofthe primary PK parameters and their 90% confidence intervals (CIs) were calculated. The two preparations were considered bioequivalent if the 90% CIs of GMRs of the primary PK parameters were within the predefined acceptance range of 80–125%.
Results
Subject characteristics
In fasting group, 30 randomized participants (28 males and 2 females) enrolled and completed the study. The demographic details, mean ± SD (range): age, 27.83 ± 6.25 years (18.0–38.0 years); height, 170.25 ± 5.79 cm (159–179.5 cm); weight, 62.87 ± 7.88 kg (46–77 kg); body mass index (BMI), 21.67 ± 2.3 kg/m2 (18.1–26 kg/m2). In fed group, 29 randomized participants (21 males and 8 females) enrolled and completed the study. One subject voluntarily withdrew before high-fat meal, and the other subject withdrew due to intolerance to high-fat meal. The demographic details, mean ± SD (range): age, 25.72 ± 6.52 years (18–44 years); height, 167.9 ± 7.81 cm (153–182 cm); weight, 61.66 ± 7.82 kg (48–77 kg); BMI, 21.87 ± 2.31 kg/m2 (8.6–26 kg/m2). Thus, all subjects who received study drugs were included in the safety analysis set and PK analysis set.
Pharmacokinetics
All subjects completed the study and the data were included in the pharmacokinetic analysis. The mean plasma concentration versus time profiles of ezetimibe and ezetimibe glucuronide under fasting and fed conditions are illustrated in Figs. 1,2,3 and 4. The pharmacokinetic parameters were summarized in Table 1. The 90% CIs for the geometric mean ratios (GMRs) of Cmax, AUC0–t, and AUC0-∞, and the power under fasting and fed conditions were presented in Table 2.
Mean plasma concentration versus time profiles of ezetimibe (A), ezetimibe glucuronide (B), and total ezetimibe (C) under fasting conditions, following a single dose of the test (T, 10 mg ezetimibe tablets) and reference (R, 10 mg Ezetrol®) in Chinese subjects. Error bars represent standard deviations, n = 30
Mean plasma concentration versus time profiles of ezetimibe (A), ezetimibe glucuronide (B), and total ezetimibe (C) under fed conditions, following a single dose of the test (T, 10 mg ezetimibe tablets) and reference (R, 10 mg Ezetrol®) in Chinese subjects. Error bars represent standard deviations, n = 29. a Mean plasma concentration-time curves of ezetimibe. b Mean plasma concentration-time curves of ezetimibe glucuronide .c Mean plasma concentration-time curves of total ezetimibe
In the fasting study, compared with the reference preparation, the Cmax, AUC0–t, and AUC0-∞ of ezetimibe of the test preparation was 94.89, 97.56 and 98.09% respectively;the Cmax, AUC0–t, and AUC0-∞ of ezetimibe glucuronide of the test preparation was 91.3, 96.59 and 95.78% respectively; the Cmax, AUC0–t, and AUC0-∞ of total ezetimibe (ezetimibe+ ezetimibe glucuronide) of the test preparation was 91.05, 97.14 and 98.13% respectively. These ratios were within the predefined equivalence limit of 80 ~ 125%.These 90% CIs all fell within the range of 80.00% ~ 125.00%.
In the fed study, compared with the reference preparation, the Cmax, AUC0–t, and AUC0-∞ of ezetimibe of the test preparation was 96.28, 92.05 and 95.04% respectively; the Cmax, AUC0–t, and AUC0-∞ of ezetimibe glucuronide of the test preparation was 96.60, 90.12 and 95.03% respectively, the Cmax, AUC0–t, and AUC0-∞ of total ezetimibe (ezetimibe+ ezetimibe glucuronide) of the test preparation was 97.10, 90.50 and 92.84% respectively. These 90% CIs all fell within the range of 80.00% ~ 125.00%.
Safety
During the whole study period, both ezetimibe tablet and Ezetrol® showed good tolerance. In In the study of fasting condition, a total of 8 volunteers increasing in urinary red blood cells, leukocyte, and neutrophil, sore throat, and epistaxis were observed in 15 times AEs tests. These adverse reactions were considered to be related with the study drugs. In the study of fed condition, 3 volunteers increasing in urine leukocytosis and anemia were detected in 3 times AEs. None of them were judged as serious adverse events (SAEs).
Discussion
According to FDA guidelines [11], the bioequivalence of ezetimibe tablets in healthy adults was evaluated by 90% CI of ezetimibe and total ezetimibe. In our study, both under fasting and fed conditions, the bioavailability of ezetimibe and total ezetimibe were calculated by Cmax, AUC0–t and AUC0-∞, and the 90% CI fell between 80.00–125.00%. The 90% CI calculated by AUC0-∞ fell between 0.80–1.25, indicating that the test preparation and the reference preparation are equivalent in absorption degree, metabolism degree and peak concentration. Tmax of ezetimibe, ezetimibe glucuronide and total ezetimibe were similar in each condition, indicating that absorption rates had no statistically significant difference under fasting condition and fed condition. T1/2 and λz, were essentially the same for the two components in both studies.
There is a statistical difference in the incidence of adverse events between the reference and the test preparation, which may be caused by comprehensive factors such as individual differences and environment, but there is no difference in the incidence of adverse reactions. Therefore, it cannot be explained that the two preparations are not equivalent. Ezetimibe tablets have good tolerance and safety under the dose conditions of this study.
These results indicated that the Ezetrol®/ezetimibe tablets were bioequivalent and exchangeable in clinical practice. Therefore, ezetimibe tablet could be further developed to become a convenient option to treat high cholesterol in Chinese patients.
Conclusions
In conclusion, the test ezetimibe tablets developed by Chongqing shenghuaxi Pharmaceutical Co., Ltd. are equivalent to the Ezetrol® produced by MSD Pharma (Singapore) Pte. Ltd. The results confirmed the PK of ezetimibe tablets to be similar to that of the Ezetrol® after a single dose of 10 mg in healthy chinese participants. The trial proved that ezetimibe tablets and Ezetrol® were bioequivalent under both fasted condition and fed condition. The ezetimibe tablets can be used in the treatment of exogenous cholesterol absorption in the patients.
Availability of data and materials
All of the data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- LC-MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- GCP:
-
Good clinical practice
- CIs:
-
Confidence intervals
- NMPA:
-
China National Medical Products Administration
- HIV:
-
Human immunodeficiency virus
- AEs:
-
Adverse events
- AUC:
-
Area under curve
- Cmax :
-
Maximum plasma concentration
- Tmax :
-
Time to Cmax
- T½:
-
Elimination half-life
- SD:
-
Standard deviation
- BMI:
-
Body mass index
- ECG:
-
Electrocardiogram
- SAEs:
-
Serious adverse events
References
Davidson MH, McGarry T, Bettis R, Melani L, Lipka LJ, LeBeaut AP, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40(12):2125–34. https://doi.org/10.1016/s0735-1097(02)02610-4 PMID: 12505224.
Sudhop T, Lütjohann D, Kodal A, Igel M, Tribble DL, Shah S, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106(15):1943–8. https://doi.org/10.1161/01.cir.0000034044.95911.dc PMID: 12370217.
Kosoglou T, Statkevich P, Johnson-Levonas AO, et al. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions [J]. Clin Pharmacokinet. 2005;44(5):467–94. https://doi.org/10.2165/00003088-200544050-00002.
Sweeney ME, Johnson RR. Ezetimibe: an update on the mechanism of action, pharmacokinetics and recent clinical trials [J]. Expert Opin Drug Metab Toxicol. 2007;3(3):441–50. https://doi.org/10.1517/17425255.3.3.441.
Zafrir B, Jain M. Lipid-lowering therapies, glucose control and incident diabetes: evidence, mechanisms and clinical implications [J]. Cardiovasc Drugs Ther. 2014;28(4):361–77. https://doi.org/10.1007/s10557-014-6534-9.
Park JG, Oh GT. Current pharmacotherapies for atherosclerotic cardiovascular diseases [J]. Arch Pharm Res. 2019;42(3):206–23. https://doi.org/10.1007/s12272-019-01116-1.
World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. https://doi.org/10.1001/jama.2013.281053.
Takezawa M. Good clinical practice (GCP) in clinical trials [J]. Nihon Yakurigaku Zasshi. 2011;138(5):205–8.
Li S, Liu G, Jia J, Li X, Yu C. Liquid chromatography-negative ion electrospray tandem mass spectrometry method for the quantification of ezetimibe in human plasma. J Pharm Biomed Anal. 2006;40(4):987–92. https://doi.org/10.1016/j.jpba.2005.07.053 Epub 2005 Oct 19.
European Medicines Agency. Ezetimibe tablet 10 mg product-specific bioequivalence guidance. 2020.
U.S. Food and Drug Administration. Draft Guidance on Ezetimibe. 2008.
Acknowledgements
This study would not have been possible without the cooperation of the volunteers, clinical investigators, nurses, and study coordinators. We are grateful to Chongqing shenghuaxi Pharmaceutical Co., Ltd. and Affiliated Hospital of Qingdao University.
Funding
Sponsorship for this study was funded by Chongqing shenghuaxi Pharmaceutical Co., Ltd.,and grants from the Natural science foundation of Shandong province (ZR2019MH101). The funder had no role in study design, data collection, analysis, and interpretation, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
The study was designed by YC. FFS and YPL wrote the paper, participated in data statistics and performance. TL, PPL, XJ, XL, CJW, XMG, YPM, and YF performed research and participated in the acquisition of data. The author (s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study passed the review of Medical Ethics Committee of the Affiliated Hospital of Qingdao University on June 22, 2017, and obtained the approval (No. QYFYEC 2017–026-01). The written informed consents was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors report no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, F., Liu, Y., Li, T. et al. Pharmacokinetics and bioequivalence of Ezetimibe tablet versus Ezetrol®:an open-label, randomized, two-sequence crossover study in healthy Chinese subjects. BMC Pharmacol Toxicol 24, 7 (2023). https://doi.org/10.1186/s40360-023-00649-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40360-023-00649-y