Abstract
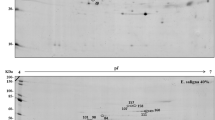
Korean fir is an endemic ornamental tree species facing population decline in Korea. To further understand the acclimatory adjustments it undergoes in response to seasonal extremes, we characterized some of the needle proteins that are upregulated during winter. Two-dimensional gel electrophoresis (2-DE), followed by MALDI TOF/TOF MS/MS and Mascot analyses, was used to visualize changes in protein profiles during acclimation to winter stress. From the 2-DE protein profiles of Korean fir needles, 226 protein spots were detected, many of which accumulated at higher levels during the winter. Among 17 proteins identified, 12 matched proteins associated with photosynthesis and with biotic and abiotic stresses, and eight were significantly upregulated under winter stress. Upregulated proteins included photosynthetic enzymes sedoheptulose-1,7-bisphosphatase and fructose bisphosphate aldolase of the Calvin–Benson cycle, four proteins related to oxidative stress tolerance, two proteins implicated in biotic defense, one heat-shock protein, and five unknown proteins. However, two other oxidative-stress-related proteins were present at high levels throughout the year, and a chitinase and the small subunit of ribulose-1,5-bisphosphate carboxylase showed no seasonal adjustments. Thus, Korean fir needles exhibited winter upregulation of some photosynthetic enzymes, coupled with increased photo protective thermal energy dissipation, and proteins related to abiotic and biotic stress resistance. Winter stress, which can include both low temperature and reduced water availability, in the subalpine region of Mount Halla led to an altered physiological equilibrium with increases in key Calvin–Benson cycle enzymes and increased enzymatic and non-enzymatic protection against oxidative stress.




Similar content being viewed by others
References
Adams WW III, Demmig-Adams B (1994) Carotenoid composition and down regulation of photosystem II in three conifer species during the winter. Physiol Plant 92:451–458
Adams WW III, Demmig-Adams B (1995) The xanthophyll cycle and sustained thermal energy dissipation activity in Vinca minor and Euonymus kiautschovicus in winter. Plant Cell Environ 18:117–127
Adams WW III, Zarter CR, Mueh KE, Amiard V, Demmig-Adams B (2006) Energy dissipation and photoinhibition: a continuum of photoprotection. In: Demmig-Adams B, Adams WW III, Mattoo AK (eds) Photoprotection, photoinhibition, gene regulation, and environment. Advances in photosynthesis and respiration, vol 21. Springer, Dordrecht, pp 49–64
Adams WW III, Demmig-Adams B, Verhoeven AS, Barker DH (1995a) ‘Photoinhibition’ during winter stress: involvement of sustained xanthophyll cycle-dependent energy dissipation. Aust J Plant Physiol 22:261–276
Adams WW III, Hoehn A, Demmig-Adams B (1995b) Chilling temperatures and the xanthophyll cycle. A comparison of warm-grown and overwintering spinach. Aust J Plant Physiol 22:75–85
Adams WWIII, Demmig-Adams B, Rosenstiel TN, Brightwell AK, Ebbert V (2002) Photosynthesis and photoprotection in overwintering plants. Plant Biol 4:545–557
Adams WW III, Cohu CM, Muller O, Demmig-Adams B (2013) Foliar phloem infrastructure in support of photosynthesis. Front Plant Sci 4:194
Adams WW III, Stewart JJ, Cohu CM, Muller O, Demmig-Adams B (2016) Habitat temperature and precipitation of Arabidopsis thaliana ecotypes determine the response of foliar vasculature, photosynthesis, and transpiration to growth temperature. Front Plant Sci 7:1026
Bartoli CG, Casalongué CA, Simontacchi M, Marquez-Garcia B, Foyer CH (2013) Interactions between hormone and redox signalling pathways in the control of growth and cross tolerance to stress. Environ Exp Bot 34:73–88
Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cho HK, Miyamoto T, Takahashi K, Hong SG, Kim JJ (2007) Damage to Abies koreana seeds by soil-borne fungi on Mount Halla, Korea. Can J For Res 37:371–382
Cohu CM, Muller O, Stewart JJ, Demmig-Adams B, Adams WW III (2013) Association between minor loading vein architecture and light- and CO2-saturated photosynthetic oxygen evolution among Arabidopsis thaliana ecotypes from different latitudes. Front Plant Sci 4:264
Cui S, Huang F, Wang J, Ma X, Cheng Y, Liu J (2005) A proteomic analysis of cold stress responses in rice seedlings. Proteomics 5:3162–3172
Demmig-Adams B, Adams WW III (2006) Photoprotection in an ecological context: the remarkable complexity of thermal dissipation. New Phytol 172:11–21
Demmig-Adams B, Cohu CM, Muller O, Adams WW III (2012) Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth Res 113:75–88
Demmig-Adams B, Stewart JJ, Adams WW III (2014) Multiple feedbacks between chloroplast and whole plant in the context of plant adaptation and acclimation to the environment. Phil Trans R Soc B 369:20130244
Duffy CDP, Chmeliov J, Macernis M, Sulskus J, Valkunas L, Ruban AV (2013) Modeling of fluorescence quenching by lutein in the plant light-harvesting complete LHCII. J Phys Chem B 117:10974–10986
Ekramoddoullah AKM, Yu X, Sturrock R, Zamani A, Taylor D (2000) Detection and seasonal expression pattern of a pathogenesis-related protein (PR-10) in Douglas fir (Pseudotsuga menziesii) tissues. Physiol Plant 110:240–247
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155:93–100
Foyer CH, Ruban AV, Noctor G (2017) Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem J 474:877–883
Galindo-González LM, El Kayal W, Morris JS, Cooke JEK (2015) Diverse chitinases are invoked during the activity-dormancy transition in spruce. Tree Genet Genomes 11:41
Harris GC, Antoine V, Chan M, Nevidomskyte D, Königer M (2006) Seasonal changes in photosynthesis, protein composition and mineral content in Rhododendron leaves. Plant Sci 170:314–325
Hirayama T, Shinozaki K (2010) Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J 61:1041–1052
Horton P (2012) Optimization of light harvesting and photoprotection: molecular mechanisms and physiological consequences. Phil Trans R Soc B 367:3455–3465
Hoshino T, Xiao N (2009) Cold adaptation in the phytopathogenic fungi causing snow molds. Mycoscience 50:26–38
Ilioaia C, Johnson MP, Liao PN, Pascal AA, van Grondelle R, Walla PJ, Ruban AV, Robert B (2011) Photoprotection in plants involves a change in lutein 1 binding domain in the major light-harvesting complex of photosystem II. J Biol Chem 286:27247–27254
Ilioaia C, Duffy CDP, Johnson MP, Ruban AV (2013) Changes in the energy transfer pathways within photosystem II antenna induced by xanthophyll cycle activity. J Phys Chem B 117:5841–5947
IUCN (2013) IUCN Red List of Threatened Species. Version 2013. 2. http://www.iucnredlist.org. Accessed 7 September 2010
Jacob P, Hirt H, Bendahmane A (2017) The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotech J 15:405–414
Kang YJ, Kim SC, Kim WW, Kim CS, Park YB (1990) Variation of cone and needle characteristics of Abies koreana along the elevation gradients in Mt. Halla. Res Rep Inst For Gen Korea 26:119–123
Kjellsen TD, Shiryaeva L, Schöder WP, Strimbeck GR (2010) Proteomics of extreme freezing tolerance in Siberian spruce (Picea obovata). J Proteomics 73:965–975
Kocsy G, Tari I, Vanková R, Zechmann B, Gulyás Z, Poór P, Galiba G (2013) Redox control of plant growth and development. Plant Sci 211:77–91
Koh SC, Demmig-Adams B, Adams WW III (2009) Novel patterns of seasonal photosynthetic acclimation, including interspecific differences, in conifers over an altitudinal gradient. Arct Antarct Alp Res 41:317–322
Kopczewski T, Kuzniak E (2013) Redox signals as a language of interorganellar communication in plant cells. Cent Eur J Biol 8:1153–1163
Kosová K, Vítámvás P, Prášil IT, Renaut J (2011) Plant proteome changes under abiotic stress: contribution of proteomics studies to understanding plant stress response. J Proteomics 74:1301–1322
Kuwabara C, Imai R (2009) Molecular basis of disease resistance acquired through cold acclimation in overwintering plants. J Plant Biol 52:19–26
Lee TB (1986) Endemic plants and their distribution in Korea. J Natl Acad Sci Korea 21:169–218
Lee WT (1996) Lineamenta Florae Koreae. Academy Press, Seoul, p 113
Lee DH, Lee IC, Kim KJ, Kim DS, Na HJ, Lee I-J, Kang S-M, Jeon H-W, Le PY, Ko J-H (2014) Expression of gibberellin 2-oxidase 4 from Arabidopsis under the control of a senescence-associated promoter results in a dominant semi-dwarf plant with normal flowering. J Plant Biol 57:106–116
Liu J-J, Ekramoddoullah AKM (2004) Characterization, expression and evolution of two novel subfamilies of Pinus monticola cDNAs encoding pathogenesis-related (PR)-10 proteins. Tree Physiol 24:1377–1385
Liu J-J, Ekramoddoullah AKM, Yu X (2003) Differential expression of multiple PR10 proteins in western white pine following wounding, fungal infection and cold-hardening. Physiol Plant 119:544–553
Logan BA, Kornyeyev D, Hardison J, Holaday AS (2006) The role of antioxidant enzymes in photoprotection. Photosynth Res 88:119–132
Matsumoto N (1994) Ecological adaptations of low temperature plant pathogenic fungi to diverse winter climates. Can J Plant Pathol 16:237–240
Oakley BR, Kirsch DR, Morris NR (1980) A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem 105:361–363
Oh SJ, Koh JG, Kim ES, Oh MY, Koh SC (2001) Diurnal and seasonal variation of chlorophyll fluorescence from Korean fir plants on Mt. Halla. Kor J Environ Biol 19:43–48
Oh SJ, Adams WW III, Demmig-Adams B, Koh SC (2013) Seasonal photoprotective responses in needles of Korean fir (Abies koreana) over an altitudinal gradient on Mount Halla, Jeju Island, Korea. Arct Antarct Alp Res 45:238–248
Ruban AV, Johnson MP, Duffy CDP (2012) The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta 1817:167–181
Saravana RS, Rose JKC (2004) A critical evaluation of sample extraction techniques for enhanced proteomic analysis of recalcitrant plant tissues. Proteomics 4:2522–2532
Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem 68:850–858
Skyba M, Petijova L, Kosuth J, Koleva DP, Ganeva TG, Kapchina-Toteva VM, Cellarova E (2012) Oxidative stress and antioxidant response in Hypericum perforatum L. plants subjected to low temperature treatment. J Plant Physiol 169:955–964
Stone JK, Hood A, Watt MS, Kerrigan JL (2007) Distribution of Swiss needle cast in New Zealand in relation to winter temperature. Aust Plant Pathol 36:445–454
Sundar D, Chaitanya KV, Jutur PP, Reddy AR (2004) Low temperature induced changes in antioxidative metabolism in rubber-producing shrub, guayule (Parthenium argentatum Gray). Plant Growth Regul 44:175–181
Sung DY, Vierling E, Guy CL (2001) Comprehensive expression profile analysis of the Arabidopsis HSP70 gene family. Plant Physiol 126:789–800
Thayer S, Björkman O (1990) Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth Res 23:331–343
Ukaji N, Kuwabara C, Takezawa D, Arakawa K, Fujikawa S (2004) Accumulation of pathogenesis-related (PR) 10/Bet v 1 protein homologues in mulberry (Morus bombycis Koidz.) tree during winter. Plant, Cell Environ 27:1112–1121
Verhoeven AS, Adams WW III, Demmig-Adams B (1996) Close relationship between the state of the xanthophyll cycle pigments and photosystem II efficiency during recovery from winter stress. Physiol Plant 96:567–576
Wäli PR, Helander M, Nissinen O, Saikkonen K (2006) Susceptibility of endophyte-infected grasses to winter pathogens (snow molds). Can J Bot 84:1043–1051
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Roles of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Woo SY, Lim JW, Lee DK (2008) Effects of temperature on photosynthetic rates in Korean fir (Abies koreana) between healthy and dieback population. J Integr Plant Biol 50:129–152
Xi J, Wang X, Li S, Zhou X, Yue L, Fan J, Hao D (2006) Polyethylene glycol fractionation improved detection of low-abundant proteins by two-dimensional electrophoresis analysis of plant proteome. Phytochemistry 67:2341–2348
Xing WB, Rajashekar CB (2001) Glycine betaine involvement in freezing tolerance and water stress in Arabidopsis thaliana. Environ Exp Bot 46:21–28
Yamazaki JY, Tsuchiya S, Nagano S, Maruta E (2007) Photoprotective mechanisms against winter stress in the needles of Abies mariesii grown at the tree line on Mt. Norikura in Central Japan. Photosynthetica 45:547–554
Yamori W, Hikosaka K, Way DA (2014) Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynth Res 119:101–117
Yan JQ, Wang J, Zhang H (2002) An ankyrin repeat-containing protein plays a role in both disease resistance and antioxidation metabolism. Plant J 29:193–202
Zarter CR, Adams WW III, Ebbert V, Cuthbertson D, Adamska I, Demmig-Adams B (2006a) Winter downregulation of intrinsic photosynthetic capacity coupled with upregulation of Elip-like proteins and persistent energy dissipation in a subalpine forest. New Phytol 172:272–282
Zarter CR, Demmig-Adams B, Ebbert V, Adamska I, Adams WW III (2006b) Photosynthetic capacity and light harvesting efficiency during the winter-to-spring transition in subalpine conifers. New Phytol 172:283–292
Zarter CR, Adams WW III, Ebbert V, Adamska I, Jansson S, Demmig-Adams B (2006c) Winter acclimation of PsbS and related proteins in the evergreen Arctostaphylos uva-ursi as influenced by altitude and light environment. Plant, Cell Environ 29:869–878
Zeng Y, Yu J, Cang J, Liu LJ, Mu YC, Wang JH, Zhang D (2011) Detection of sugar accumulation and expression levels of correlative key enzymes in winter wheat (Triticum aestivum) at low temperatures. Biosci Biotech Bioch 75:681–687
Zhang Q, Zhang JZ, Chow WS, Sun LL, Chen JW, Chen YJ (2011) The influence of low temperature on photosynthesis and antioxidant enzymes in sensitive banana and tolerant plantain (Musa sp.) cultivars. Photosynthetica 49:201–208
Zhang W, Zhao X, Shi M, Yang A, Hua B, Liu Y (2016) Altered protein expression in peach (Prunus persica) following fruit bagging. Korean J Hortic Sci Technol 34:32–45
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0361) and the University of Colorado.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, S., Adams, W.W., Demmig-Adams, B. et al. A proteomic approach to seasonal adjustment in the enzyme complement of Korean fir (Abies koreana Wilson) needles. Hortic. Environ. Biotechnol. 60, 135–146 (2019). https://doi.org/10.1007/s13580-018-0094-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-018-0094-z