Abstract
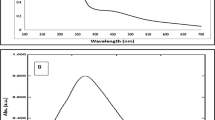
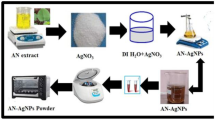
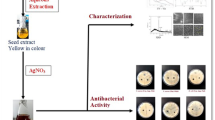
In this study, seed and seed shell extracts of Cola nitida were investigated for the biogenic synthesis of silver nanoparticles (AgNPs) under ambient condition. The biosynthesized AgNPs were characterized through visual development of colour, UV–vis spectroscopy, Fourier transform infrared spectroscopy (FTIR) and transmission electron microscopy (TEM). The antibacterial activities of the AgNPs were determined using some multidrug-resistant clinical isolates. The biosynthesized AgNPs depicted brown and yellowish orange colour using seed and seed extract, respectively, with maximum absorbance readings at 457.5 and 454.5 nm. The FTIR spectrum showed strong peaks at 3292.49, 2086.98 and 1631.78 cm−1 for seed extract-mediated AgNPs, while peaks of 3302.13, 2086.05 and 1633.71 cm−1 were obtained for seed shell extract-mediated AgNPs, all indicating that proteins were the capping and stabilization molecules in the biogenic synthesis of AgNPs. The AgNPs were spherical in shape with sizes ranging from 8 to 50 and 5 to 40 nm for seed and seed shell-mediated AgNPs, respectively. The energy-dispersive X-ray (EDX) analysis showed the presence of silver as a prominent metal, while the selected area electron diffraction (SAED) pattern conformed to the face-centred cubic phase and crystalline nature of AgNPs. At various concentrations ranging from 50–150 μg/ml, the AgNPs inhibited growth of multidrug-resistant strains of Klebsiella granulomatis, Pseudomonas aeruginosa and Escherichia coli to the tune of 10–32 mm. Comparatively, seed shell extract-mediated AgNPs had better activities with minimum inhibitory concentration (MIC) of 50 μg/ml against all the tested isolates, while the MICs of seed extract-mediated AgNPs were obtained as 50, 80 and 120 μg/ml against E. coli, P. aeruginosa (wound) and P. aeruginosa (burn), respectively. This study has demonstrated the feasibility of eco-friendly biogenic synthesis of AgNPs using seed and seed shell extracts of C. nitida, and the report to the best of our knowledge is the first reference to extracts of C. nitida for the green synthesis of AgNPs.









Similar content being viewed by others
References
Lateef, A., Adelere, I. A., Gueguim-Kana, E. B. (2015). Bacillus safensis LAU 13: a new source of keratinase and its multi-functional biocatalytic applications. Biotechnology and Biotechnological Equipment, 29, 54–63.
Lateef, A., Adelere, I. A., Gueguim-Kana, E. B. (2015). The biology and potential biotechnological applications of Bacillus safensis. Biologia, 70, 411–419.
Lateef, A., Adelere, I. A., Gueguim-Kana, E. B., Asafa, T. B., Beukes, L. S. (2015). Green synthesis of silver nanoparticles using keratinase obtained from a strain of Bacillus safensis LAU 13. International Nano Letters, 5, 29–35.
Lateef, A., Ojo, S.A., Azeez, M.A., Asafa, T.B., Yekeen, T.A., Akinboro, A., Oladipo, I.C., Gueguim-Kana, E.B., Beukes, L.S. (2015). Cobweb as novel biomaterial for the green and eco-friendly synthesis of silver nanoparticles. Applied Nanoscience, doi: 10.1007/s13204-015-0492-9.
Salem, W. M., Haridy, M., Sayed, W. F., Hassan, N. H. (2014). Antibacterial activity of silver nanoparticles synthesized from latex and leaf extract of Ficus sycomorus. Industrial Crops and Products, 62, 228–234.
Shivaji, S., Madhu, S., Singh, S. (2011). Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochemistry, 46, 1800–1807.
Augustine, R., Kalarikkal, N., Thomas, S. (2014). A facile and rapid method for the black pepper leaf mediated green synthesis of silver nanoparticles and the antimicrobial study. Applied Nanoscience, 4, 809–818.
Kumar, B., Smita, K., Cumbal, L., Debut, A. (2014). Sacha inchi (Plukenetia volubilis L.) oil for one pot synthesis of silver nanocatalyst: an ecofriendly approach. Industrial Crops and Products, 58, 238–243.
Mishra, A., Kumari, M., Pandey, S., Chaudhry, V., Gupta, K. C., Nautiyal, C. S. (2014). Biocatalytic and antimicrobial activities of gold nanoparticles synthesized by Trichoderma sp. Bioresource Technology, 166, 235–242.
Nazeruddin, G. M., Prasad, N. R., Prasad, S. R., Shaikh, Y. I., Waghmare, S. R., Adhyapak, P. (2014). Coriandrum sativum seed extract assisted in situ green synthesis of silver nanoparticle and its anti-microbial activity. Industrial Crops and Products, 60, 212–216.
Shanmugam, N., Rajkamal, P., Cholan, S., Kannadasan, N., Sathishkumar, K., Viruthagiri, G., et al. (2014). Biosynthesis of silver nanoparticles from the marine seaweed Sargassum wightii and their antibacterial activity against some human pathogens. Applied Nanoscience, 4, 881–888.
El-Batal, A. I., ElKenawya, N. M., Yassin, A. S., Amin, M. A. (2015). Laccase production by Pleurotus ostreatus and its application in synthesis of gold nanoparticles. Biotechnology Reports, 5, 31–39.
Yallappa, S., Manjanna, J., Dhananjaya, B. L. (2015). Phytosynthesis of stable Au, Ag and Au–Ag alloy nanoparticles using J. sambac leaves extract, and their enhanced antimicrobial activity in presence of organic antimicrobials. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 137, 236–243.
Agharkar, M., Kochrekar, S., Hidouri, S., Azeez, M. A. (2014). Trends in green reduction of graphene oxides, issues and challenges: a review. Material Research Bulletin, 59, 323–328.
Akinoso, R., Aremu, A. K., Balogun, I. S. (2014). Some physical properties of kola nuts—a response surface approach. International Agrophysics, 28, 251–255.
Odebode, A. C. (1996). Phenolic compounds in the kola nut (Cola nitida and Cola acuminata) (Sterculiaceae) in Africa. Revista de Biología Tropical, 44, 513–515.
Asogwa, E. U., Anikwe, J. C., Ihokwunye, F. C. (2006). Kola production and utilization for economic development. African Scientist, 7, 4–5.
Dewole, E. A., Dewumi, D. F. A., Alabi, J. Y. T., Adegoke, A. (2013). Proximate and phytochemical of Cola nitida and Cola acuminata. Pakistan Journal of Biological Science, 2013, 1–4.
Jarvis, G. (2002). The rise and fall of cocaine cola. https://www.lewrockwell.com/2002/05/gail-jarvis/the-rise-and-fall-of-cocaine-cola/ (2002). Accessed 25 April, 2015.
Lowe, H. I. C., Watson, C. T., Badal, S., Peart, P., Toyang, N. J., Bryant, J. (2014). Promising efficacy of the Cola acuminata plant: a mini review. Advances in Biological Chemistry, 4, 240–245.
Orwa, C., Mutua, A., Kindt, R., Jamnadass, R., Anthony, S. (2009). Agroforestry tree database: a tree reference and selection guide version 4.0 (http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp). Accessed on 19 June, 2015.
Edison, T. J. I., & Sethuraman, M. G. (2012). Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochemistry, 47, 1351–1357.
Muniyappan, N., & Nagarajan, N. S. (2014). Green synthesis of silver nanoparticles with Dalbergia spinosa leaves and their applications in biological and catalytic activities. Process Biochemistry, 49, 1054–1061.
Kumar, S. S., Venkateswarlu, P., Rao, V. R., Rao, G. N. (2013). Synthesis, characterization and optical properties of zinc oxide nanoparticles. International Nano Letters, 3, 30–36.
Rajeshkumar, S., Ponnanikajamideen, M., Malarkodi, C., Malini, M., Annadurai, G. (2014). Microbe-mediated synthesis of antimicrobial semiconductor nanoparticles by marine bacteria. Journal of Nanostructure and Chemistry, 4, 96–102.
Gopinath, K., Venkatesh, K. S., Ilangovan, R., Sankaranarayanan, K., Arumugam, A. (2013). Green synthesis of gold nanoparticles from leaf extract of Terminalia arjuna, for the enhanced mitotic cell division and pollen germination activity. Industrial Crops and Products, 50, 737–742.
Fayaz, A. M., Balaji, K., Girilal, M., Yadav, R., Kalaichelvam, P. T., Venketesan, R. (2010). Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomedicine: Nanotechnology, Biology and Medicine, 6, 103–109.
Niraimathi, K. L., Sudha, V., Lavanya, R., Brindha, P. (2013). Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Colloids and Surfaces B: Biointerfaces, 102, 288–291.
Khanna, A. S. (2008). Nanotechnology in high performance paint coatings. Asian Journal of Experimental Science, 21(2), 25–32.
Cho, K. H., Park, J. E., Osaka, T., Park, S. G. (2005). The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochimica Acta, 51, 956–960.
Mahendra, R., Alka, Y., Aniket, G. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances, 27(1), 76–83.
Bhat, R., Deshpande, R., Ganachari, S. V., Huh, D. O., Venkataraman, A. (2011). Photo-irradiated bio-synthesis of silver nanoparticles using edible mushroom Pleurotus florida and their antibacterial activity studies. Bioinorganic Chemistry and Applications, 2011, 1–7.
Lateef, A., Davies, T. E., Adelekan, A., Adelere, I. A., Adedeji, A. A., Fadahunsi, A. H. (2010). Akara Ogbomoso: microbiological examination and identification of hazards and critical control points. Food Science and Technology International, 16, 389–400.
Lateef, A., Ojo, M.O. (2015). Public health issues in the processing of cassava (Manihot esculenta) for the production of ‘lafun’ and the application of hazard analysis control measures. Quality Assurance and Safety of Crops and Foods, doi: 10.3920/QAS2014.0476.
Chortyk, T. O., Severson, R. F., Cutler, H. C., Siesson, V. A. (1993). Antibiotic activities of sugar esters isolated from selected Nicotiana species. Bioscience Biotechnology and Biochemistry, 57, 1355–1356.
Andrews, J. M. (2005). BSAC standardized disc susceptibility testing method (version 4). Journal of Antimicrobial Chemotheraphy, 56, 60–76.
Kalishwaralal, K., Deepak, V., Ramkumarpandian, S., Nellaiah, H., Sangiliyandi, G. (2008). Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Material Letters, 62, 4411–4413.
Shaligram, N. S., Bule, M., Bhambure, R., Singhal, R. S., Singh, S. K., Szakacs, G., et al. (2009). Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochemistry, 44, 939–943.
Thirumurugan, A., Tomy, N. A., Kumar, H. P., Prakash, P. (2011). Biological synthesis of silver nanoparticles by Lantana camara leaf extracts. International Journal of Nanomaterials and Biostructure, 1(2), 22–24.
Zaki, S., El-Kady, M. F., Abd-El-Haleem, D. (2011). Biosynthesis and structural characterization of silver nanoparticles from bacterial isolates. Material Research Bulletin, 46, 1571–1576.
Kannan, R. R. R., Arumugam, R., Ramya, D., Manivannan, K., Anantharaman, P. (2013). Green synthesis of silver nanoparticles using marine macroalga Chaetomorpha linum. Applied Nanoscience, 3, 229–233.
Priyadarshini, S., Gopinath, V., Priyadharsshini, N. M., Ali, D. M., Velusamy, P. (2013). Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its application. Colloids and Surfaces B: Biointerfaces, 102, 232–237.
Shankar, S., Jaiswal, L., Aparna, R. S. L., Prasad, V. (2014). Synthesis, characterization, in vitro biocompatibility, and antimicrobial activity of gold, silver and gold silver alloy nanoparticles prepared from Lansium domesticum fruit peel extract. Material Letters, 137, 75–78.
Shameli, K., Ahmad, M. B., Zargar, M., Wan Yunus, W. M. Z., Ibrahim, N. A., Sha-banzadeh, P., et al. (2011). Synthesis and characterization of silver/montmorillonite/chitosan bionanocomposites by chemical reduction method and their antibacterial activity. International Journal of Nanomedicine, 6, 271–284.
Adewoye, S. O., & Lateef, A. (2004). Assessment of the microbiological quality of Clarias gariepinus exposed to an industrial effluent in Nigeria. The Environmentalist, 24, 249–254.
Lateef, A. (2004). The microbiology of a pharmaceutical effluent and its public health implications. World Journal of Microbiology and Biotechnology, 20, 167–171.
Lateef, A., Oloke, J. K., Gueguim-Kana, E. B. (2004). Antimicrobial resistance of bacterial strains isolated from orange juice products. African Journal of Biotechnology, 3, 334–338.
Lateef, A., Oloke, J. K., Gueguim-Kana, E. B. (2005). The prevalence of bacterial resistance in clinical, food, water and some environmental samples in Southwest Nigeria. Environmental Monitoring and Assessment, 100, 59–69.
Lateef, A., Oloke, J. K., Gueguim-Kana, E. B., Pacheco, E. (2006). The microbiological quality of ice used to cool drinks and foods in Ogbomoso Metropolis, Southwest, Nigeria. Internet Journal of Food Safety, 8, 39–43.
Lateef, A., Yekeen, T. A., Ufuoma, P. E. (2007). Bacteriology and genotoxicity of some pharmaceutical wastewaters in Nigeria. International Journal of Environmental Health, 1(4), 551–562.
Lateef, A., & Yekeen, T. A. (2006). Microbial attributes of a pharmaceutical effluent and its genotoxicity on Allium cepa. International Journal of Environmental Studies, 63(5), 534–536.
Kanmani, P., & Lim, S. T. (2013). Synthesis and structural characterization of silver nanoparticles using bacterial exopolysaccharide and its antimicrobial activity against food and multidrug resistant pathogens. Process Biochemistry, 48, 1099–1106.
Acknowledgments
AL thanked authority of LAUTECH, Ogbomoso, Nigeria, for the provision of some of the facilities used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lateef, A., Azeez, M.A., Asafa, T.B. et al. Cola nitida-Mediated Biogenic Synthesis of Silver Nanoparticles Using Seed and Seed Shell Extracts and Evaluation of Antibacterial Activities. BioNanoSci. 5, 196–205 (2015). https://doi.org/10.1007/s12668-015-0181-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-015-0181-x