Abstract
Green synthesis of nanoparticles is widely accepted due to the less toxicity in comparison with chemical methods. But there are certain drawbacks like slow formation of nanoparticles, difficulty to control particle size and shape make them less convenient. Here we report a novel cost-effective and eco-friendly method for the rapid green synthesis of silver nanoparticles using leaf extracts of Piper nigrum. Our results suggest that this method can be used for obtaining silver nanoparticles with controllable size within a few minutes. The fabricated nanoparticles possessed excellent antibacterial property against both Gram-positive and Gram-negative bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reducing the particle size of materials is an efficient tool for improving their bioactivity. A number of preparation methods have been described for the synthesis of metallic nanoparticles (Pal et al. 2007a; Rosemary and Pradeep 2003); such as, reverse micelles process (Xie et al. 2006; Maillard et al. 2002), salt reduction (Pillai and Kamat 2004), microwave dielectric heating reduction (Patel et al. 2005), ultrasonic irradiation (Salkar et al. 1999), radiolysis (Karim et al. 2007; Remita et al. 2007), solvothermal synthesis (Starowicz et al. 2006), electrochemical synthesis (Zhu et al. 2001; Liu et al. 2001) and biological techniques (Naik et al. 2002). The most widespread method of synthesis of metallic nanoparticles is based on the chemical reduction of metal salt solution by a reducing agent (Szczepanowicz et al. 2010). But the chemicals used in such processes are quite often toxic and flammable which make them unsuitable for many applications including biological applications like impregnation in wound dressings (Maneerung et al. 2008; Vivekanandhan et al. 2012) and sutures (Augustine and Rajarathinam 2012).
Biological syntheses of nanoparticles are not only a good way to fabricate benign nanostructure materials, but also to reduce the use or generation of hazardous substances to human health and the environment (Justin Packia Jacob et al. 2012). These biological methods are regarded as safe, cost-effective, sustainable and environment friendly as well as they do not require any special culture preparation and isolation techniques (Gardea et al. 2003). Biosynthesis of silver nanoparticles using microorganisms like bacteria (Saifuddin et al. 2009), fungi (Duran et al. 2009) and yeast (Kowshik et al. 2003) are already reported. However, exploitation of the plant extracts as the potential agents for the biosynthesis of nanoparticles has opened a new way—the green synthesis of nanoparticles. Further, synthesis of silver nanoparticles using extracts of various plants like Aloe vera (Chandran et al. 2006), Cinnamon zeylanicum (Sathishkumar et al. 2009), Stevia rebaudiana (Varshney et al. 2010), Papaya (Jain et al. 2009), Geranium (Shiv Shankar et al. 2008), Cassia angustifolia (Peter Amaladhas et al. 2012), Ocimum tenuiflorum (Patil et al. 2012), lemon (Vankar and Shukla 2012), carob (Awwad et al. 2013), Coleus aromaticus (Vanaja and Annadurai 2012), were also reported.
Piper nigrum Linn. or Black pepper is a perennial woody climbing liana belonging to the family Piperaceae. It is native to India, Indonesia, Malaysia, South America and West Indies but is also widely cultivated in the tropical regions. It is considered as the ‘King of Spices’ (Srinivasan 2007; Mathew et al. 2001). The presence of a pungent alkaloid (Tripathi et al. 1996) piperine (C17H19NO3) attributes a spicy taste to the seeds, leaves and other parts of Piper (Khajuria et al. 2002). It also contains small amounts of safrole (C10H10O2), pinene (C10H16), sabinene (C10H16), limonene (C10H16), caryophyllene (C15H24) and Linalool (C10H18O) compound. The fruits have variety of activities including CNS depressant, antipyretic, analgesic, hepatoprotective (Lee et al. 1984), bioavailability enhancer (Zhao et al. 2007; Kasibhatta and Naidu 2007), antioxidant (Khajuria et al. 1997) and anti-inflammatory (Majumdar et al. 1990).
Antioxidants are compounds that can delay or inhibit the oxidation of lipids or other molecules by inhibiting the initiation or propagation of oxidative chain reaction (Velioglu et al. 1998; Chanwitheesuk et al. 2005). From the P. nigram, a number of such compounds have been isolated and characterized. Alkaloids such as pellitorine (C14H25NO), (E)-1-[3’,4’-(methylenedioxy)cinnamoyl]piperidine (C15H17NO3), 2,4-tetradecadienoic acid isobutyl amide (C16H25NO), piperine (C17H19NO3), cepharadione A (C18H11NO4), piperolactam D (C17H13NO4) and paprazine (C17H17NO3) have been isolated from black pepper (Ee et al. 2009). Wei et al. (2004) isolated some amide-containing alkaloids from P. nigrum. They are Pipercycliamide (C14H26NO3), (±)-erythro-1-(1-oxo-4,5-dihydroxy-2E-decaenyl)piperidine (C15H28NO3), (±)-threo-1-(1-oxo-4,5-dihydroxy-2E-decaenyl)piperidine (C15H28NO3), (±)-threo-N-isobutyl-4,5-dihydroxy-2E-octanamide (C12H23NO3Na), 1-(1,6-dioxo-2E,4E-decadienyl)piperidine (C15H23NO2), 1-1-oxo-3(3,4-methylenedioxy-5-methoxyphenyl)-2Z-propenylpiperidine (C16H19NO4), and 1-1-oxo-5(3,4-methylenedioxyphenyl)-2Z,4E-pentadienylpyrrolidine (C16H17–NO3) (Wei et al. 2004). A number of novel dimeric amide alkaloids possessing a cyclobutane or a cyclohexene ring termed as nigramides have also been isolated from the roots of P. nigrum (Wei et al. 2005). Shanmugapriya et al. (2012) extracted alkaloids, phenolic compounds, saponins and flavonoids from the leaves of black pepper. They have also shown the antioxidant potential of these compounds. These alkaloids may act as reducing agents, free radical scavengers or potential complexers of pro-oxidant metals (Hudson 1990). Thus as in the chemical reduction process, these natural reducing agents may act upon silver nitrate and reduce it into metallic silver nanoparticles.
Even though the biological synthesis of nanoparticles is advantageous in many aspects, the long duration needed for the formation of nanoparticles makes it inconvenient. Many workers tried to develop a method for the rapid biosynthesis of various kinds of nanoparticles but all these attempts took more than 15 min to get nanoparticles (Darroudi et al. 2010; Shenya et al. 2012; Philip 2010; Praveen Kumar et al. 2011; Bar et al. 2009). Further the controllability of the size of the nanoparticles was poor in previous reports. Thus in the present study, we report a green method for the synthesis of silver nanoparticles using an aqueous leaf extract of P. nigram and no toxic chemicals are used as reducing and stabilizing agents during the synthesis.
Materials and methods
Materials
Silver nitrate of analytical grade purchased from Sigma Aldrich was used as a starting material without further purification. The P. nigram leaves were freshly collected from the agricultural field near Kottayam, Kerala, India.
Preparation of the leaf extract
Around 5 g of freshly collected P. nigram leaves were grinded well using mortar and pestle. 100 ml of deionized water was added into the slurry and filtered through a cheese cloth. The filtrate was again filtered through Whatman No. 1 filter paper (pore size 25 μm). The fresh filtrate was used for the present study.
Synthesis of silver nanoparticles
1–5 mM aqueous solutions of silver nitrate (AgNO3) was prepared in double distilled water and used for the synthesis of silver nanoparticles. 100 ml aqueous solution of the silver nitrate solution was taken in a round bottom flask and heated to boiling with a magnetic stirrer. The flask was covered with aluminum foil to prevent light-mediated reduction of silver nitrate. Then around 5 ml of the P. nigram leaf extract was added drop wise into the silver nitrate solution. During this process solution was mixed vigorously. Within 1 min the color change was evident (pale red). Then it was removed from the heating mantle and stirred until cooled to room temperature. A portion of the obtained suspensions were used for UV–Visible spectroscopic analysis. Remaining part was centrifuged at 12,000 rpm several times in distilled water and finally in ethanol to get pure silver nanoparticles.
UV–Vis spectra analysis
The optical properties (absorbance) of colloidal solution were evaluated using a Shimadzu double-beam spectrophotometer (model 1700) at a resolution of 1 nm, between 200 and 600 nm. The reduction of pure Ag+ ions was monitored by measuring the UV–Vis spectrum of the reaction medium after diluting a small aliquot of the sample into distilled water.
FTIR (Fourier-transform IR) analysis
FTIR studies on the samples were carried out using Perkin Elmer, Spectrum 400 to ensure the formation of silver nanoparticles. FTIR measurements help to identify the possible interactions between silver and bioactive molecules, which may be responsible for formation and stabilization (capping material) of silver nanoparticles.
TEM analysis of silver nanoparticles
To understand the morphology and size distribution, the synthesized nanoparticles were imaged using JEOL JEM 2100 high resolution transmission electron microscope. The sample was prepared by air-drying drops of diluted solutions of the preparations on carbon films supported by copper grids. The particle size was measured using ImageJ software and the average particle size was determined.
XRD analysis
XRD was recorded in the 2θ range of 30–80° using D8-Advance of Bruker (Germany), of CuKα radiation, the energy of which was 8.04 keV and wavelength was 1.54 Å. The applied voltage was 40 kV and current was 25 mA.
Antimicrobial activity
The disc-diffusion assay Kirby–Bauer method (Pal et al. 2007b) was used by many workers to determine the growth inhibition of bacteria by the silver nanoparticles (Sadeghi et al. 2010; Augustine and Rajarathinam 2012; Amany and El-Rab 2012). The bacterial strains Escherichia coli (ATCC 12228) and Staphylococcus aureus (ATCC6538-P) were used as representatives of Gram-negative and Gram-positive bacteria, respectively. The bacteria were cultured in Mueller–Hinton Broth (MHB) at 37 °C and prepared to the turbidity equivalent to 0.5 McFarland standards (McFarland 1907). Then 100 μl of the bacterial suspension was spread on the nutrient agar. Sterile blank discs with 6 mm diameter (HiMedia, Mumbai) were impregnated with silver nanoparticles synthesized at different silver nitrate concentrations so that to get a final nanoparticle concentration of 10 μg/disc. These were then placed on the surface of the test plate. A standard antibiotic disc was used as positive control (Ciprofloxacin, 10 μg/disc). The culture plates were incubated for overnight in an incubator at 37 °C. The diameters of the inhibition zones were measured in millimeters (mm). The experiment was repeated for three times to get an average value.
Results and discussion
Color change
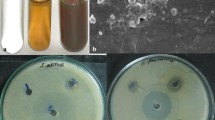
From the visual color change we can get preliminary information regarding the formation of silver nanoparticles. As the silver nanoparticles are formed, the color of the solution changes from white to pale yellow to brick red which is an indication of the presence of silver nanoparticles (Fig. 1). The variation of the color was due to the change in surface plasmon resonance of silver nanoparticles during the formation. At a silver nitrate concentration of 1 mM a very pale yellow color was obtained. While increasing the concentration of silver nitrate up to 5 mM, there is an increase in the redness of the solution. This is due to the fact that as the concentration of silver nitrate increases, aggregation of formed silver ions occurs and which leads to the formation of larger sized silver nanoparticles.
UV–Visible spectrum
Characteristic absorption peaks of silver nanoparticles can be seen at around 400–475 nm. Figure 2 shows the UV–Visible spectra which are recorded after the completion of the reaction. For 1 mM solution, silver nanoparticles has shown absorbance maxima at 440 nm, 2 mM solution has peak at 445 nm, 2 mM solution has at 445 nm, 3 mM has at 448 nm, 4 mM has at 463 nm, and 5 mM solution has at 476 nm. Electrons are limited to specific vibrations modes by the particle’s size and shape. The frequency and width of the surface plasmon absorption depend on the size and shape of the metal nanoparticles (Wang et al. 2007) as well as on the dielectric constant of the metal itself and the surrounding medium (Rai et al. 2006). Here the medium dielectric constant and temperature can be considered as the same but the mean diameter and morphology of the nanoparticles strongly affect the SPR band in aqueous solution (Wiley et al. 2006). The spectrum shows the red shift with increasing the molar concentration of silver nitrate. It indicates the increase of mean diameter of the silver nanoparticles as the concentration increases (Rai et al. 2006; Song and Kim 2009; Fayaz et al. 2009).
FTIR
The dried nanoparticle samples were analyzed in FTIR to identify the possible bio molecules responsible for the reduction of the Ag+ ions by Piper leaf extract. The FTIR spectrum is presented in Fig. 3.
The FTIR absorption spectra of unreduced silver nitrate and silver nanoparticles after the reduction and capping by Piper extract were taken. While comparing the FTIR spectra of unreduced silver nitrate and biosynthesized silver nanoparticles, there is a notable reduction in certain peaks and appearance of certain new peaks was observed. Absorbance bands in pure silver nitrate were observed in the region of 450–1,750 cm−1 and are 1,584, 1,287, 799, 731 and 639 cm−1. These absorbance bands are associated with the nitro compounds. A broad peak at 1,287 cm−1 which is present in the spectrum of silver nitrate is not found in the spectrum of silver nanoparticles. It indicates the loss of nitro group from silver species during the reduction process. Molecules containing NO2 groups, such as nitro compounds, nitrates, and nitramines, commonly exhibit asymmetric and symmetric stretching vibrations of the NO2 group at 1,660–1,500 and 1,390–1,260 cm−1 region. Apart from these losses of functional moieties, a number of new peaks found in the case of biosynthesized silver nanoparticles.
The FTIR spectrum of Ag nanoparticles showed distinct peaks 1,643 cm−1, which represent the involvement of C–N in plane vibrations, 1,035 and 1,217 cm−1 shows the involvement of C–N in plane vibrations of aliphatic amines. The peak 1,514 cm−1 arises from N–H in-plane bending and C–N stretching modes. It may be due to the C–N bond present in the amide-containing alkaloids attached to the silver nanoparticles, which make the synthesized nanoparticles stable. Peak at 1,736 cm−1 is an indication of C=O Stretch and probably due to ketones. Further the presence of C–O Stretch in between 1,300 and 1,000 cm−1 may be due to the covalent linking of ester or ether groups to the nanoparticle. Esters show their carbonyl C=O stretch at 1,750–1,735 cm−1, but also exhibit their characteristic absorption at 1,300–1,000 cm−1 from the couplings of C–O and C–C stretches. Thus, the peak at 1,035 cm−1 indicates the coupling of C–O and C–C stretches and 1,736 and 1,643 cm−1 indicates C=O stretch. Hence, there may be an ester link with the silver nanoparticle. These observations indicate the presence and binding of certain amide-containing compounds with silver nanoparticles. It may be due to the binding of one or more amide-containing alkaloids which is present in Piper like nigramides, N-isobutyl-4-hexanoyl-4-hydroxypyrrolidin-1-one, (±)-erythro-1-(1-oxo-4,5-dihydroxy-2E-decaenyl)piperidine, (±)-threo-1-(1-oxo-4,5-dihydroxy-2E-decaenyl)piperidine, (±)-threo-N-isobutyl-4,5-dihydroxy-2E-octanamide, 1-(1,6-dioxo-2E,4E-decadienyl)piperidine, 1-1-oxo-3(3,4-methylenedioxy-5-methoxyphenyl)-2Z-propenylpiperidine, 1-1-oxo-5(3,4-methylenedioxyphenyl)-2Z, 4E-pentadienylpyrrolidine, etc., to the synthesized silver nanoparticles. This may be the possible reason for the stability of synthesized silver nanoparticles.
TEM
Transmission electron microscopy provided a clear idea about the morphology and size of the synthesized silver nanoparticles. Comparison of TEM results has shown that the diameter of prepared nanoparticles in the solution was about 5–50 nm. Figures 4, 5, 6, 7 and 8 show the transmission electron micrograph of silver nanoparticles obtained from the proposed bioreduction method at various silver nitrate concentrations. At a silver nitrate concentration of 1 mM, silver nanoparticles with spherical morphology is obtained (Fig. 4). Each nanoparticle is individually remained without forming any agglomeration. The average particle size at this silver nitrate concentration is 8 nm. At a silver nitrate concentration of 2 mM almost similar morphology obtained as in the case of 1 mM concentration but the average particle diameter increased to 12 nm (Fig. 5). When the silver nitrate concentration was further increased, the size of the nanoparticles tends to decrease. Agglomeration of nanoparticles also observed at higher silver nitrate concentrations. At 3 mM silver nitrate concentration nanoparticles with almost spherical morphology were obtained with an average particle size of 18 nm (Fig. 6). The heterogeneity in particle size can be observed here. At 4 and 5 mM silver nitrate concentrations, the morphology of the nanoparticles becomes irregular higher tendency for agglomeration could be observed (Figs. 7, 8). At 5 mM silver nitrate concentration the control over the particles size seemed to be completely lost and nanoparticles with varying diameters in the range of 5–50 nm obtained.
XRD
The XRD result (Fig. 9) shows that the silver nanoparticles formed are crystalline. A number of strong Bragg reflections can be seen which correspond to the (111), (200), (220), (311) reflections of face-centered cubic (fcc) silver. This is in agreement with the results obtained by other workers (Parikh et al. 2011; Theivasanthi and Alagar 2011; Martinez-Castanon et al. 2008). A sharp and strong diffraction peak centered at 38° was appeared, which can be indexed to the (1 1 1) reflection of the metallic silver with fcc structure (JCPDS File No. 04-0783). Comparably weak diffraction peaks at 44°, 66° and 77° in the pattern agree well with the (2 0 0), (2 2 0) and (3 1 1) reflections, respectively. The broadening of the peaks clearly indicates that the particles formed are in the nanoscale.
Antimicrobial activity
The disc diffusion assay or Kirby–Bauer method (Pal et al. 2007b), which is employed to assess preliminary antimicrobial activity of antimicrobial agents, was used to evaluate the antibacterial activity of silver nanoparticles synthesized at different silver nitrate concentrations. Silver nanoparticles synthesized at all the silver nitrate concentrations displayed antibacterial activity against both E. coli and S. aureus (Fig. 10). The diameter of inhibition zones around the disc containing silver nanoparticles in E. coli and S. aureus are given in Table 1. Nanoparticles prepared using 1 mM silver nitrate was the most efficient to inhibit both E. coli and S. aureus. Then there is a decrease in antibacterial activity as the concentration of silver nitrate increased. This can be correlated to the increase in particle size as the concentration of silver nitrate increased during synthesis. Antibacterial activity of silver nanoparticles was found to be dependent on the size of silver particles and as the size increases the antibacterial activity decreases (Panáček et al. 2006). All the synthesized nanoparticles have shown more antibacterial activity against S. aureus than E. coli as reported by other workers (Sadeghi et al. 2010; Shrivastava et al. 2007), probably due to the difference in cell walls between Gram-positive and Gram-negative bacteria. Moreover, the synthesized nanoparticles enhance the therapeutic efficacy and strengthen the medicinal values of this plant. Antibacterial activity of black pepper (P. nigrum Linn.) and its mode of action on both Gram-negative and Gram-positive bacteria were already reported (Karsha and Lakshmi 2010).
Antimicrobial activity of synthesized silver nanoparticles against E. coli (plate a) and S. aureus (plate b). In both cases nanoparticles synthesized at 1, 2, 3, 4 and 5 mM silver nitrate concentrations are denoted by a, b, c, d and e, respectively. In both plates f denotes standard antibiotic disc (Ciplofloxacin)
Conclusions
A simple and facile process has been described for the preparation of silver nanoparticles starting from silver nitrate as a precursor and leaf extract of P. nigram as reducing as well as stabilizing agent. The characterization from UV–Visible, FTIR and TEM data supports the stability of the biosynthesized nanoparticles. The average particle size and morphology of the synthesized nanoparticles has been found to depend on the molar concentration of silver nitrate solution used. The FTIR studies indicate the capping of certain amide-containing compounds on the synthesized nanoparticles. The XRD analysis of the powder indicates that the synthesized nanoparticles are crystalline with fcc structure. The results obtained in this study are comparable with that obtained while chemical reducing agents like citrate of sodium or sodium borohydrate are used.
References
Amaladhas Peter, Sivagami S, Akkini Devi T, Ananthi N, Priya Velammal S (2012) Biogenic synthesis of silver nanoparticles by leaf extract of Cassia angustifolia. Adv Nat Sci Nanosci Nanotechnol 3(4):045006
Amany A, El-Rab SFG (2012) Effect of reducing and protecting agents on size of silver nanoparticles and their anti-bacterial activity. Der Pharma Chemica 4(1):53–65
Augustine R, Rajarathinam K (2012) Synthesis and characterization of silver nanoparticles and its immobilization on alginate coated sutures for the prevention of surgical wound infections and the in vitro release studies. Int J Nano Dimens 2(3):205–212
Awwad AM, Salem NM, Abdeen AO (2013) Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int J Ind Chem 4(1):1–6
Bar H, Bhui DK, Sahoo GP, Sarkar P, Pyne S, Misra A (2009) Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf A Physicochem Eng Aspects 348:212–216
Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M (2006) Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog 22:577–583
Chanwitheesuk A, Teerawutgulrag A, Rakariyatham N (2005) Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem 92:491–497
Darroudi M, Ahmad MB, Abdullah AH, Ibrahim NA, Shameli K (2010) Effect of accelerator in green synthesis of silver nanoparticles. Int J Mol Sci 11:3898–3905
Duran N, Marcato PD, De Souza GIH (2009) Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J Biomed Nanotechnol 5:247–253
Ee GCL, Lim CM, Lim CK, Rahmani M, Shaari K, Bong CFJ (2009) Alkaloids from Piper sarmentosum and Piper nigrum. Nat Prod Res 23:1416–1423
Fayaz AM, Balaji K, Kalaichelvan PT, Venkatesan R (2009) Fungal based synthesis of silver nanoparticles—an effect of temperature on the size of particles. Colloids Surf B 74:123–126
Gardea-Torresdey JL, Gomez E, Peralta-Videa J, Parsons JG, Troiani HE, Jose-Yacaman M (2003) Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir 19:357–1361
Hudson BJF (1990) Food antioxidants. Elsevier Applied Science, London, pp 65–98
Jain D, Kumar Daima H, Kachhwaha S, Kothari SL (2009) Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their antimicrobial activities. Dig J Nanomater Biostruct 4:557–563
Justin Packia Jacob S, Finub JS, Narayanan A (2012) Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surf B Biointerfaces 91:212–214
Karim MR, Lim KT, Lee CJ, Bhuiyan MTI, Kim HJ, Park L-S, Lee MS (2007) Synthesis of core-shell silver–polyaniline nanocomposites by gamma radiolysis method. J Polym Sci Part A Polym Chem 45:5741–5747
Karsha P, Lakshmi VOB (2010) Antibacterial activity of black pepper (Piper nigrum Linn.) with special reference to its mode of action on bacteria. Indian J Nat Prod Resour 1:213–215
Kasibhatta R, Naidu MU (2007) Influence of piperine on the pharmacokinetics of nevirapine under fasting conditions: a randomized, crossover placebo-controlled study. Drugs RD 8:83–139
Khajuria A, Thusus N, Zutshi U, Bedi KL (1997) Antioxidant potential of piperine on oxidant induced alterations in rat intestinal lumen. Indian Drugs 34:557–563
Khajuria A, Thusu N, Zutshi U (2002) Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultra-structure and enzyme kinetics. Phytomedicine 9(3):224–231
Kowshik M, Ashtaputre S, Kharrazi S, Vogel W, Urban J, Kulkarni SK, Paknikar KM (2003) Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 14:95–100
Lee EB, Shin KH, Woo WS (1984) Central nervous system depressant and anti-inflammatory activity of piperine. Arch Pharmacol Res 7:127–132
Liu S, Chen S, Avivi S, Gendanken A (2001) Synthesis of X-ray amorphous silver nanoparticles by the pulse sonoelectrochemical method. J Non Cryst Solids 283:231–236
Maillard M, Giorgo S, Pileni MP (2002) Silver nanodisks. Adv Mater 14(15):1084–1086
Majumdar AM, Dhuley JN, Deshmukh VK, Raman PH, Naik SR (1990) Antiinflammatory activity of piperine. Jpn J Med Sc Biol 43:95–100
Maneerung T, Seiichi T, Ratana R (2008) Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr polym 72(1):43–51
Martinez-Castanon GA, Nino-Martinez N, Martinez-Gutierrez F, Martinez-Mendoza JR, Ruiz F (2008) Synthesis and antibacterial activity of silver nanoparticles with different sizes. J Nanopart Res 10(8):1343–1348
Mathew PJ, Mathew PM, Kumar V (2001) Graph clustering of Piper nigram L. (black pepper). Euphytica 118:257–264
McFarland J (1907) The nephelometer: an instrument for estimating the number of bacteria in suspensions for calculating the opsonic index and vaccines. J Am Med Assoc 49:1176–1178
Naik RR, Stringer SJ, Agarwal G, Jones S, Stone MO (2002) Biomimetic synthesis and patterning of silver nanoparticles. Nat Mater 1(3):169–172
Pal A, Shah S, Devi S (2007a) Preparation of silver, gold and silver–gold bimetallic nanoparticles in w/o microemulsion containing TritonX-100, colloids and surfaces. Physicochem Eng Aspects 302:483–487
Pal S, Kyung Y, Song Myong (2007b) J Appl Environ Microbiol 73(6):1712–1720
Panáček A, Kvitek L, Prucek R, Kolar M, Vecerova R, Pizurova N, Sharma VK, Nevečná T, Zboril R (2006) Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B 110(33):16248–16253
Parikh RY, Ramanathan R, Coloe PJ, Bhargava SK, Patole MS, Shouche YS, Bansal V (2011) Genus-wide physicochemical evidence of extracellular crystalline silver nanoparticles biosynthesis by Morganella spp. PLoS One 6(6):e21401
Patel K, Kapoor S, Dave DP, Mukherjee T (2005) Synthesis of nanosized silver colloids by microwave dielectric heating. J Chem Sci 117:53–60
Patil RS, Kokate MR, Kolekar SS (2012) Bioinspired synthesis of highly stabilized silver nanoparticles using Ocimum tenuiflorum leaf extract and their antibacterial activity. Spectrochimica Acta Part A Mol Biomol Spectrosc 91:234
Philip Daizy (2010) Rapid green synthesis of spherical gold nanoparticles using Mangiferaindica leaf. Spectrochimica Acta Part A Mol Biomol Spectrosc 77:807–810
Pillai ZS, Kamat PV (2004) What factors control the size and shape of silver nanoparticles in the citrate ion reduction method? J Phys Chem B 108:945–951
Praveen Kumar K, Paul W, Chandra Sharma P (2011) Green synthesis of gold nanoparticles with Zingiber officinale extract: characterization and blood compatibility. Process Biochem 46:2007–2013
Rai AK, Singh R, Singh KN, Singh VB (2006) FTIR, Raman spectra and ab initio calculations of 2-mercaptobenzothiazole. Spectrochim Acta A Mol Biomol Spectrosc 63(2):483–90
Remita S, Fontaine P, Lacaze E, Borensztein Y, Sellame H, Farha R, Rochas C, Goldmann M (2007) X-ray radiolysis induced formation of silver nano-particles: a SAXS and UV–Visible absorption spectroscopy study. Nucl Instrum Methods Phys Res B 263:436–440
Rosemary MJ, Pradeep T (2003) Solvothermal synthesis of silver nanoparticles from thiolates. J Colloid Interface Sci 268:81–84
Sadeghi B, Jamali M, Kia S, Amini NA, Ghafari S (2010) Synthesis and characterization of silver nanoparticles for antibacterial activity. Int J Nano Dimens 1(2):119–124
Saifuddin N, Wong CW, Nur Yasumira AA (2009) Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. Eur J Chem 6:61–70
Salkar RA, Jeevanandam P, Aruna ST, Koltypin Y, Gedanken A (1999) The sonochemical preparation of amorphous silver nanoparticles. J Mater Chem 9:1333–1335
Sathishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun YS (2009) Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf B Biointerfaces 73:332–338
Shanmugapriya K, Saravana PS, Payal H, Mohammed P, Williams SB (2012) Antioxidant potential of pepper (Piper nigrum Linn.) leaves and its antimicrobial potential against some pathogenic microbes. Indian J Nat Prod Resour 3(4):570–577
Shenya DS, Philip Daizy, Mathew Joseph (2012) Rapid green synthesis of palladium nanoparticles using the dried leaf of Anacardium occidentale. Spectrochim Acta Part A Mol Biomol Spectrosc 91:35–38
Shiv Shankar S, Ahmad A, Sastry M (2008) Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog 19(6):1627–1631
Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnol 18(22):225103
Song JY, Kim BS (2009) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng 32:79–84
Srinivasan K (2007) Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Nutr 47:735–748
Starowicz M, Stypula B, Banaoe J (2006) Electrochemical synthesis of silver nanoparticles. Electrochem Commun 8:227–230
Szczepanowicz K, Stefańska J, Socha PR, Warszyński P (2010) Preparation of silver nanoparticles via chemical reduction and their antimicrobial activity. Physicochem Probl Miner Process 45:85–98
Theivasanthi T, Alagar M (2011) Electrolytic synthesis and characterizations of silver nanopowder (arXiv preprint, arXiv:1111.0260)
Tripathi AK, Jain DC, Kumar S (1996) Secondary metabolites and their biological and medical activities of Piper species plants. J Med Aromat Plant Sci 18:302–321
Vanaja M, Annadurai G (2012) Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl Nanosci 3:217–223
Vankar PS, Shukla D (2012) Biosynthesis of silver nanoparticles using lemon leaves extract and its application for antimicrobial finish on fabric. Appl Nanosci 2(2):163–168
Varshney R, Bhadauria S, Gaur MS (2010) Biogenic synthesis of silver nanocubes and nanorods using sundried Stevia rebaudiana leaves. Adv Mater Lett 1:232–237
Velioglu YS, Mazza G, Gao L, Oomah BD (1998) Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 46:4113–4117
Vivekanandhan S, Christensen L, Misra M, Mohanty AK (2012) Green process for impregnation of silver nanoparticles into microcrystalline cellulose and their antimicrobial bionanocomposite films. J Biomater Nanobiotechnol 3:371–376
Wang G, Shi Ch, Zhao N, Du X (2007) Synthesis and characterization of Ag nanoparticles assembled in ordered array pores of porous anodic alumina by chemical deposition. Mater Lett 61:3795–3797
Wei K, Li W, Koike K, Pei Y, Chen Y, Nikaido T (2004) New amide alkaloids from the roots of Piper nigrum. J Nat Prod 67:1005–1009
Wei K, Li W, Koike K, Chen Y, Nikaido T (2005) Nigramides A-S, dimeric amide alkaloids from the roots of Piper nigrum. J Org Chem 70:1164–1176
Wiley BJ, Im SH, Li ZY, McLellan J, Siekkinen A, Xia Y (2006) Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J Phys Chem B 110:15666–15675
Xie Y, Ye R, Liu H (2006) Synthesis of silver nanoparticles in reverse micelles stabilized by natural biosurfactant. Colloids Surf A 279:175–178
Zhao JQ, Du GZ, Xiong YC, Wen YF, Bhadauria M, Nirala SK (2007) Attenuation of beryllium induced hepatorenal dysfunction and oxidative stress in rodents by combined effect of gallic acid and piperine. Arch Pharm Res 30:1575–1578
Zhu JJ, Liao XH, Zhao XN, Chen HY (2001) Preparation of silver nanorods by electrochemical methods. Mater Lett 49:91–95
Acknowledgments
The authors acknowledge Department of Science and Technology (DST), Government of India, New Delhi for the financial support through Nanomission and PURSE programs. Funding from UGC SAP, DST-FIST are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Augustine, R., Kalarikkal, N. & Thomas, S. A facile and rapid method for the black pepper leaf mediated green synthesis of silver nanoparticles and the antimicrobial study. Appl Nanosci 4, 809–818 (2014). https://doi.org/10.1007/s13204-013-0260-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-013-0260-7