Abstract
Background
Recombinant human follitropin alfa (r-hFSH) is used for ovarian stimulation as part of medically assisted reproduction. There is a risk for ovarian hyperstimulation syndrome (OHSS) with r-hFSH treatment, and an increased risk for thromboembolic events in the presence of pregnancy with OHSS.
Objectives
To report the frequency of OHSS and thromboembolism with originator follitropin alfa (GONAL-f) based on the Global Safety Database of Merck KGaA, Darmstadt, Germany and a systematic review of published data.
Data Sources
Reports of OHSS and thromboembolism were obtained from the Global Safety Database of Merck KGaA, Darmstadt, Germany from 20 October 1995 to 19 October 2018. The systematic review was based on MEDLINE and Embase searches from inception to 19 October 2018.
Study Eligibility Criteria
Patients receiving GONAL-f for ovulation induction or ART, with a starting dose within the range included in the prescribing information and providing information on the occurrence of OHSS and/or thromboembolism.
Study Appraisal and Synthesis Matches
In the Global Safety Database of Merck KGaA, Darmstadt, Germany there were an estimated 16,525,975 treatment cycles since 20 October 1995; 1110 reported cases of OHSS and 80 reported cases of thromboembolic events (reporting rates 6.7 and 0.48 per 100,000 treatment cycles, respectively). The systematic review identified 45 studies (5186 patients exposed to GONAL-f; 5240 treatment cycles). There were 272 reports of OHSS (5190 [5.19%] per 100,000 treatment cycles), including 10 cases of severe OHSS (191 [0.19%] per 100,000 treatment cycles).
Limitations
There may be the potential for under-reporting of safety outcomes in the literature, and under-reporting is a well-known phenomenon in spontaneous reporting databases.
Conclusion and Implications of Key Findings
Our analyses demonstrate low rates of OHSS and thromboembolism with GONAL-f.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
More than 20 years of internal data show that the adverse drug reaction reporting rates for every 100,000 treatments started is 6.7 (0.007%) for ovarian hyperstimulation syndrome (OHSS) and 0.48 (0.0005%) for thromboembolic events. |
In the published literature, the adverse drug reaction rates for every 100,000 treatments started are 5190 (5.19%) for OHSS and 191 (0.191%) for thromboembolism. |
Of the 1110 OHSS cases reported in the Global Safety Database of Merck KGaA, Darmstadt, Germany, 130 were classified as severe; of the 272 OHSS cases retrieved from the systematic review searches, 10 cases were classified as severe. |
Treatment with GONAL-f results in low rates of thromboembolism and OHSS. |
Digital Features
This article is published with digital features to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12967055.
Introduction
Recombinant human follitropin alfa (r-hFSH) is indicated for the treatment of women with anovulation and for ovarian stimulation as part of assisted reproductive technologies (ART) (Table 1) [1,2,3]. It is also indicated for the stimulation of spermatogenesis in men with congenital or acquired hypogonadotropic hypogonadism in combination with human chorionic gonadotropin (hCG); however, this review focusses on data for use in women only.
The prescribing information for r-hFSH suggests that the starting dose should commonly be 75–150 IU daily for the treatment of women with anovulation and 150–225 IU daily for ovarian stimulation as part of ART [1,2,3]. The starting dose of r-hFSH is individualized according to patient characteristics and biomarkers [including age, smoking status, serum anti-Müllerian hormone (AMH) level, antral follicle count (AFC) and previous response to treatment] with the aim of optimizing safety and efficacy. The prescribing information also indicates that to ensure safe use of r-hFSH, ovarian response should be monitored on a regular basis (by ultrasound and measurement of serum oestradiol levels). This is because, owing to its mechanism of action and the aim of ovulation induction and controlled ovarian stimulation, there is a risk for ovarian hyperstimulation syndrome (OHSS) [4,5,6]. To mitigate the risk of OHSS and maximize the probability of a safe, fresh embryo transfer after ovarian stimulation for ART treatment in patients with potential/expected ovarian hyper-response, a starting dose of less than 150 IU of r-hFSH has been recommended [7,8,9,10,11], based on patient characteristics and biomarkers [12,13,14,15]. Individualization of the starting dose can be calculated using a nomogram developed on the basis of a woman’s age and two markers of ovarian reserve (AMH or AFC and FSH) [7, 16] or other nomograms that include factors such as body mass index (BMI) [9] or additional ultrasound markers [11]. For example, to avoid cycle cancellation or freeze-all cycles due to excessive ovarian response and related OHSS risk, in expected high responders younger than 35 years old presenting with an AMH of 7.7 ng/mL the r-hFSH starting dose calculated using the existing nomograms would be either 100–112.5 IU [7], 75–100 IU [9] or 100–125 IU [11], all of which are lower, as recommended before [8, 13], than a standard dose of 150 IU [14]. There is also a risk for thromboembolic events, which is increased in the presence of pregnancy and OHSS. Recent reports of the occurrence of OHSS and thromboembolism in different populations have reported rates of less than 0.5% [4], meaning these adverse events (AEs), although important, occur rarely during ovarian stimulation with r-hFSH [4, 17, 18].
Other AEs mentioned in the prescribing information for r-hFSH include, multiple pregnancy, pregnancy loss, ectopic pregnancy, reproductive system neoplasms and congenital newborn malformation; however, these do not directly relate to the mechanism of action of r-hFSH [1,2,3]. To reduce the risk of any adverse drug reactions (ADRs) or to avoid a cycle with no oocytes retrieved, the dose may be adjusted during treatment based on this regular monitoring.
Originator r-hFSH (GONAL-f®; Merck KGaA, Darmstadt, Germany) was the first r-hFSH product on the market, receiving approval from the European Medicines Agency (EMA) on 20 October 1995 [19], and is now available in more than 120 countries, including the USA, where it is marketed as GONAL-f RFF® (EMD Serono). With more than 20 years of use in clinical practice, GONAL-f has a large amount of available data, providing a good overview of its safety. It is important that post-marketing safety data are monitored and available in the public domain to enable clinicians and patients to evaluate potential treatment risks. The provision of all available data is especially important as a considerable amount of safety data in the scientific literature are from randomized controlled trials (RCTs), which may not reflect actual real-world clinical practice, owing to limitations including those related to their strict inclusion and exclusion criteria and short duration of follow-up.
The current manuscript presents the reporting rates of OHSS and thromboembolism with GONAL-f®. To be as comprehensive as possible, internal and external sources of information were used for this review. This should enable the assessment of the benefit–risk balance of this drug in routine clinical practice.
Methods
The frequency of reports of ADRs was evaluated in both the Global Safety Database of Merck KGaA, Darmstadt, Germany (Adverse Reaction Information System global, ARISg; ArisGlobal, Coral Gables, FL, USA) and through systematic review of the scientific literature to collect safety data on GONAL-f. These two approaches were selected because they should provide two different, but complementary, perspectives of the safety of GONAL-f: the overall view using all of the data collected by Merck KGaA, Darmstadt, Germany and the view that would be obtained by a person reading the literature. Owing to the different nature of the data, the search of the Global Safety Database of Merck KGaA, Darmstadt, Germany and the systematic review of the literature were conducted and will be presented separately. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Global Safety Database of Merck KGaA, Darmstadt, Germany Search
The Global Safety Database of Merck KGaA, Darmstadt, Germany comprises data for ADRs from clinical trials, non-interventional studies, market research, patient or healthcare provider surveys, patient support programs sponsored by Merck KGaA, Darmstadt, Germany, EMD Serono or its affiliates or investigators, spontaneous post-marketing reports and surveillance of the published literature. The literature search strategy covers, amongst others, the following aspects: adverse effects in general, toxicity, poisoning; drug addiction/misuse/abuse/doping; drug overdose; medication error/administration error; lack of efficacy; drug interaction; and off-label use. Surveillance of the scientific literature is performed on a weekly basis to supplement the safety database with relevant literature that reports important identified and potential risks (MEDLINE and Embase databases were searched comprehensively using various compilations of free text search terms, including active ingredients and respective brand names, and index terms, controlled terms from the database thesauri) (Appendix 1 in the electronic supplementary material).
Reports of OHSS and thromboembolism were obtained from the Global Safety Database of Merck KGaA, Darmstadt, Germany from 20 October 1995 to 19 October 2018. The database search was conducted using Medical Dictionary for Regulatory Activities (MedDRA®) version 21.0 [20] Preferred Term (PT) 10033266—Ovarian hyperstimulation syndrome and the Standardized MedDRA Query (SMQ) 20000081—Embolic and thromboembolic events. There were no exclusion criteria for the search of the Global Safety Database of Merck KGaA, Darmstadt, Germany. Although OHSS can be reported as mild, moderate or severe, severity is often not reported and only overall OHSS is therefore reported.
Reporting rates were calculated by dividing the number of cases reported by the estimated number of treatment cycles that occurred since the product’s international birth date (IBD; 20 October 1995). The reporting rate could not be based on the number of patients exposed to r-hFSH, owing to only data on number of units sold being available, and not number of patients exposed to r-hFSH. It is difficult to estimate the number of treated women from the sales volume, as the dose and duration of treatment are adjusted according to patient’s response to the treatment and thus may vary from one patient to another. Each treatment cycle is generally viewed as an independent cycle as treatment modifications are often made in subsequent cycles according to the ovarian response to gonadotropin stimulation in previous treatment cycles. Therefore, the treatment cycle is considered to be the most relevant denominator for calculating the patient’s exposure. The number of ovarian stimulation treatment cycles was calculated from the estimated average r-hFSH use per treatment cycle and the sales data.
Statistical analyses of reporting rates are descriptive as quantitative analyses of these data were not feasible as the database includes a population of uncertain size.
Systematic Literature Search
MEDLINE and Embase were systematically searched from inception of the databases to 19 October 2018 for interventional and non-interventional clinical studies using GONAL-f. While the search terms used for the Global Safety Database of Merck KGaA, Darmstadt, Germany were specifically chosen for the reporting of AEs, the search terms in the systematic review were ‘GONAL-f’ and ‘r-hFSH’ and their variants. “GONAL-f” OR “GONALf” OR “GONAL f” OR “follitropin alfa” OR “SJ-0021” OR SJ0021 OR “SJ 0021” OR “recombinant follicle stimulating hormone” OR “recombinant follicle-stimulating hormone” OR “recombinant human follicle stimulating hormone” OR “recombinant human follicle-stimulating hormone” OR “recombinant-human follicle-stimulating hormone” OR hFSH OR rhFSH OR “r-hFSH” OR “rh-FSH” OR “r-h-FSH”.
Searches were limited to titles and abstracts and were filtered for studies in humans and in the English language. Editorials, commentaries, reviews (including narrative reviews, systematic reviews and meta-analyses), lectures, letters to the editor and case series and reports were excluded, as these were anticipated to have been included in the Global Safety Database of Merck KGaA, Darmstadt, Germany. The reference lists of any meta-analyses identified were reviewed to identify any additional references that should also be considered but were missed during the search.
The inclusion criteria for studies were women with infertility receiving GONAL-f for ovulation induction or ART, with a starting dose within the range included in the prescribing information and providing information on the occurrence of OHSS and/or thromboembolism.
Abstracts were initially reviewed by a medical writer (AJ) to remove duplicates and abstracts explicitly not meeting the inclusion criteria. Subsequently, two review authors (TDH, JH) independently reviewed the remaining abstracts for relevant studies for inclusion based upon the criteria described earlier. As safety is frequently not reported in abstracts, a cautious approach was taken to abstract screening; manuscripts that might be expected to describe safety were not excluded at this point, rather they were retained for full-text screening. The same two authors (TDH, JH) then reviewed the full text of the remaining references to identify relevant studies. Discrepancies were resolved by adjudication (SL).
Data were extracted using a standard form developed by discussion with all authors (Appendix 2 in the electronic supplementary material). Data extraction was conducted by AJ and DM, and to ensure data accuracy, quality control of data extraction was repeated for a random selection of references (n = 15) by JH and checked against the data from AJ/DM. Data on the following outcomes were extracted:
-
Occurrence of OHSS overall and by grade, when reported (mild, moderate, severe, critical). The grade was based on the diagnosis provided within each study, with the study’s definition for each grade of OHSS used.
-
Occurrence of thromboembolism.
The Cochrane Collaboration’s qualitative checklist to assess the methodological quality of the included studies was independently completed by each reviewer to assess risk of bias [21]. Conflicts were adjudicated by discussion between AJ and DM, with resolution of any remaining conflicts by JH.
Results
Global Safety Database of Merck KGaA, Darmstadt, Germany Search
Overall, an estimated 16,525,975 treatment cycles were conducted between the IBD of 20 October 1995 and 19 October 2018. It was estimated that the mean dose of GONAL-f administered per treatment cycle to each woman was 1875 IU, based on clinical trial data of Merck KGaA, Darmstadt, Germany.
There were 1110 reported cases of OHSS in the database and 80 reported cases of thromboembolic events; 29 of the thromboembolic events were reported to be accompanied by OHSS. Of the 1110 OHSS cases, severity was unknown or not reported in 562 (50.1%) cases. Furthermore, 146 (13%) were reported to be mild, 269 (24%) were reported to be moderate and 130 (11.7%) were reported to be severe; this includes eight cases with life-threatening OHSS. In addition to the 130 severe cases, three fatal cases of OHSS were reported: one literature case and two cases reported by healthcare professionals. Causality was reported as possible in one case and not reported in the other two cases. One fatal case had a thromboembolic event in combination with OHSS. The source of the reports from the Global Safety Database of Merck KGaA, Darmstadt, Germany are shown in Table 2. The reporting rates for OHSS and thromboembolism were 6.7 per 100,000 (1110/16,525,975) treatment cycles (ca. 0.007%) and 0.48 (80/16,525,975) per 100,000 treatment cycles (0.0005%), respectively.
Systematic Literature Search
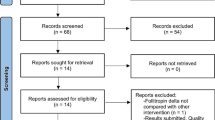
Of 2243 publications initially identified, 45 unique articles were deemed eligible for inclusion in the analysis (Fig. 1) [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66]. These reported data from 36 RCTs, eight non-randomized studies based on primary data and one non-randomized study based on secondary use of data. In total, data for 5186 patients receiving 5240 treatment cycles of ovarian stimulation with GONAL-f were included. In 4.4% (2/45) of studies OHSS was assessed as a primary outcome, in 17.8% (8/45) as a secondary outcome and in 71.1% (32/45) as a safety or other outcome (three studies did not specify OHSS as an outcome). In total, 73.3% (33/45) of the studies classified OHSS according to severity; however, the classification system or the parameters used to classify OHSS were only reported in 45.5% (15/33) of those studies. Overall 272 reported cases of OHSS (reporting rate 5190 per 100,000 treatment cycles; 5.19%) including 10 cases of severe OHSS (3.7% of all 272 OHSS case reported; reporting rate 191 per 100,000 treatment cycles; 0.19%) were identified (Table 3). There were no fatal cases of OHSS and no reports of thromboembolism were identified (Table 3).
Risk of Bias
The risk of bias was assessed as high, low or unclear/unreported in seven categories (random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessments, incomplete outcome data, selective reporting and other biases). Only five studies were judged to be free of bias in all of the categories [24, 25, 41, 55, 65]. Bias was mostly reported for allocation concealment, blinding of participants and blinding of outcome assessment (Fig. 2). However, the safety outcomes reported in these articles were deemed to be important and were taken into account. Overall the risk of bias was low and none of the selected studies were excluded owing to the level of bias.
Discussion
This manuscript reports the frequency of OHSS and thromboembolism with GONAL-f in both the Global Safety Database of Merck KGaA, Darmstadt, Germany and in the published scientific literature. GONAL-f has a long history of usage; overall, there were an estimated 18 million treatment cycles conducted between the IBD of 20 October 1995 and 19 October 2019. This is the first cumulative report of these safety events specifically for a single gonadotropin preparation. There have been other analyses, including real-world studies, evaluating the frequency of OHSS and thromboembolism; however, the majority of previous published reports described pooled data for several gonadotropins, rather than for a single preparation. It is important to analyse data for all gonadotropin preparations separately (e.g. originator r-hFSH alfa and its biosimilars, as well as for r-hFSH beta and delta), as differences have been observed with regard to biochemical properties, in vitro activity, pharmacokinetics, pharmacodynamics and reproductive outcomes observed in clinical trials [1,2,3, 67,68,69]. In addition, other external safety databases such as the Uppsala Monitoring Center Vigibase and the FDA Adverse Event Reporting System (FAERS), also do not differentiate between GONAL-f and other gonadotropin preparations, and do not provide the data on the total number of cycles or the number of patients treated, which makes it impossible to calculate frequency rates.
A large retrospective chart review conducted in 2010 (using data from 2004 onwards) reported on 22,665 cycles using r-hFSH (GONAL-f or Puregon) for the indications licensed in the summary of product characteristics [70]. OHSS (any severity) was identified in 3812/20,144 (18.9%) cycles and hospitalization due to OHSS (grade not specified) was reported in 215/20,144 (1.1%) cycles. A Cochrane review covering the period from inception to 2017, including 20 trials of 6088 women treated with r-hFSH, reported OHSS rates of 2.5% for moderate or severe OHSS and 0.8% for severe OHSS for patients treated with standard dose FSH (150 IU), with rates between 0.8% and 2.5% reported for patients for whom the dose was adjusted according to the results of ovarian reserve tests [71]. However, the studies included in the Cochrane review were heterogeneous and the evidence quality of these studies ranged from low to moderate and was potentially subject to bias. The evidence from older studies indicates that OHSS is predominantly mild to moderate, with mild cases occurring in an estimated 20–33% of ART fertility treatment cycles; moderate in 3% to 6% treatment cycles and severe in 0.2–5.0% of assisted conception cycles [72,73,74,75,76,77,78]. However, because the classification of OHSS has changed over time, any comparisons of the occurrence of OHSS in older and more recent studies should be interpreted with caution. Furthermore, there has been a steady decline of OHSS rates with the refinement of ovarian stimulation protocols with the use of gonadotropin-releasing hormone (GnRH) antagonist to prevent a premature LH surge [79] and use of a GnRH agonist to trigger final oocyte maturation [80], which might reflect more in recent studies.
There are internationally recognised classifications for OHSS, which have been updated several times over the years. The first OHSS classification was proposed in 1967 dividing OHSS into three categories (mild, moderate and severe) and with two degrees of severity for each category [81]. This was subsequently amended to include an additional category (‘critical’) and subcategories (grades A–C for severe OHSS) [81, 82]. More recently a new classification system has been proposed in which OHSS is classified as either ‘self-limited’ or ‘with significant comorbidities’ to simplify reporting [81]. The International Committee for Monitoring Assisted Reproductive Technologies (ICMART) defined OHSS as mild, moderate or severe, according to the degree of abdominal distention, ovarian enlargement and respiratory, haemodynamic and metabolic complications. The committee also included a distinct definition of severe OHSS in the glossary: ‘a systemic response as a result of ovarian stimulation interventions that is characterized by severe abdominal discomfort and/or other symptoms of ascites, hemoconcentration (Hct > 45) and/or other serious biochemical abnormalities requiring hospitalization for observation and/or for medical intervention (paracentesis, other)’ [15]. These historic changes in definitions make it difficult to compare OHSS risk over time.
The OHSS reporting rate in our systematic review is higher than in the analysis of the Global Safety Database of Merck KGaA, Darmstadt, Germany. Firstly, this may be because the reports of OHSS and thromboembolism were obtained from the Global Safety Database of Merck KGaA, Darmstadt, Germany from 20 October 1995 onwards and, although awareness of AE reporting has increased during the past decade, the period covered by the database also includes a period when there was less awareness of AE reporting. The extent of under-reporting of ADRs to spontaneous reporting systems and the differences among different types of ADRs up to April 2004 were addressed in a systematic review by Hazell and Shakir [83]. In this systematic review, the median under-reporting rate across 37 studies in 12 countries was 94% (interquartile range 82–98%), providing evidence of significant and widespread under-reporting of ADRs to spontaneous reporting systems, including serious or severe ADRs, during the first decade covered by our database. These under-reporting rates come from well-developed countries where a pharmacovigilance system is reasonably established, and under-reporting of ADRs in spontaneous databases for products that have been on the market for a long time and have well-known ADR profiles (such as GONAL-f) is recognised [83]. The reporting of AEs to GONAL-f in countries with less-developed pharmacovigilance systems may also contribute to the under-reporting. In addition, in the search strategy used in this analysis, only the PTs of OHSS were selected, meaning that cases which reported individual symptoms suggestive of mild OHSS (mild abdominal swelling, discomfort and nausea) may not be included. Further, the low reporting rate in the Global Safety Database of Merck KGaA, Darmstadt, Germany may relate to the reporting of only more serious cases of OHSS, with mild OHSS less frequently reported. Indeed, the proportion of severe OHSS cases reported out of all OHSS cases reported was about three times higher (11.7%) in the Global Safety Database of Merck KGaA, Darmstadt, Germany when compared to the systematic review (3.7%). We hypothesize that some OHSS cases occurring in daily practice may not be considered as “serious enough” to be reported in the post-marketing setting [84], whereas they would be captured systematically in a typical phase III RCT evaluating efficacy and safety. In some of these RCTs, OHSS might have been an adverse event of special interest (AESI), ensuring that serious and non-serious AEs will be collected and reported in an expedited manner. The selection criteria used for our systematic review favoured clinical trials, which are likely to have included proactive and enhanced monitoring for ADRs (often specified in study protocols and clinical research files). However, the reporting rate in our systematic review was within the range observed in other studies (which also included a mixture of study designs) [71,72,73,74].
Finally, the characteristics of the patients included in clinical trials may not fully reflect those seen in clinical practice, as it is well established that only 37% of patients treated in the real world would be eligible for inclusion in randomized clinical trials [85].
With the exception of pharmaceutical company-sponsored trials, OHSS is rarely reported as a primary (4.4% of studies included in our systematic review) or even secondary (17.8% of studies) endpoint, and there is a lack of transparency and a high degree of variability/heterogeneity on how OHSS was assessed and defined in clinical trials. Indeed, only 45.5% of the studies that we assessed used a classification system specifying which parameters were used to classify OHSS. Broad summary statements often used in publications, such as “the product was generally safe” or “it was well tolerated” do not provide clinically meaningful information and should be avoided [86]. Furthermore, there is no consistent guidance provided by health authorities, journals or scientific communities on the reporting of fertility-related AEs and ADRs in publications; therefore, there is a need for a unified system for the reporting of safety outcomes in fertility studies. A task force has therefore been set up within Merck KGaA, Darmstadt, Germany to assess the way safety outcomes are reported in fertility trials and to develop guidance that can be used in future publications. The guidance will include a comprehensive checklist of safety parameters that should be assessed and reported in fertility-related studies. Generally reporting of OHSS and other safety results in fertility publications should be in line with the clinical study protocol and the clinical study report.
There are a number of potential limitations to the analyses reported in this manuscript. The data range from 1995 to 2018 and, as previously stated, owing to changes in clinical practice, the overall data provided might not fully reflect what is observed in current practice. Our understanding of OHSS has increased in recent years, and the risk can now be greatly reduced with appropriate treatment selection: individualized choice of gonadotropin type and starting dose; dose adjustment based on hormonal and ultrasound monitoring during ovarian stimulation; choice of ovulation trigger; choice between embryo transfer in fresh stimulation cycle or delayed embryo transfer after cryopreservation of all oocytes/embryos. For this reason, data from older studies and analyses are unlikely to provide an accurate representation of OHSS risk at the current time. Ovarian stimulation should be monitored via ultrasound and serum oestradiol levels, and if multiple immature follicles or high serum oestradiol levels are observed the dose can be reduced, a coasting strategy (i.e. stopping gonadotropin treatment until serum oestradiol levels return to normal levels) employed or the cycle cancelled, depending upon the perceived risk by the treating physician/healthcare professional [82]. Current guidance is that women at high risk for a high ovarian response and related OHSS risk should be identified before treatment is started, so that an appropriate treatment strategy can be selected to mitigate the risk. Risk factors for OHSS include the presence of polycystic ovarian syndrome, a prior history of OHSS, low body weight and younger age (less than 30 years old) [87, 88]. Biomarkers may also be used to identify patients at risk for OHSS, including AFC, AMH, day 3 FSH and inhibin B [87, 89]. If the patient wishes to have a maximal probability for a fresh embryo transfer, while avoiding both cycle cancellation and freeze-all due to high risk for moderate-severe OHSS, the number of oocytes should be limited to around 15, with a maximum of 20, oocytes. In order to achieve this, the r-hFSH starting dose should be individualized on the basis of existing normograms that include patient age, AFC, AMH [7, 16] and potentially other factors, such as BMI [9] or additional ultrasound markers [11], rather than adopting a “one-size-fits-all” dosing [90] such as 100 IU [10] or 150 IU [14]. This strategy will typically result in r-hFSH starting doses lower than 150 IU, as recommended before [8, 13, 90], varying between 75 and 125 IU, as shown in the example provided in the “Introduction”. In addition, GnRH antagonists should be used, as they enable gonadotropins to be used for a shorter duration and are known to reduce the OHSS risk [8, 12].
Gonadotropin dose adjustment using small dose changes (12.5–25 IU), in combination with combined ultrasound and hormonal monitoring, during ovarian stimulation is needed to adjust for both a lower or higher than expected ovarian response [8]. In case of an increased risk for OHSS, a GnRH agonist and/or low-dose hCG can be used to trigger ovulation, followed by, if possible (and in line with patient preference), a fresh embryo transfer or (if high risk for OHSS) cryopreservation of all embryos (freeze all) followed by embryo transfer in subsequent frozen embryo transfer cycles.
Furthermore, the definitions for classification of OHSS and thromboembolism have changed during the time period that was evaluated. The cases reported in the Global Safety Database of Merck KGaA, Darmstadt, Germany were either submitted voluntarily or obtained from the literature. Case reports were excluded from the systemic literature search, which helped to avoid duplication. There may, however, be some duplication, in the sense that we cannot rule out that some cases included in the systematic review were also reported as individual cases in the Global Safety Database of Merck KGaA, Darmstadt, Germany. We anticipate that any overlap, if present, will be small and would increase the incidence of OHSS in the Global Safety Database of Merck KGaA, Darmstadt, Germany, resulting in an overestimate of the OHSS risk in this database. Although the two analyses are complementary, the reported outcomes should be considered separately to avoid overestimation of the frequency of OHSS. The number of treatment cycles in the post-marketing setting was estimated from sales data, and the number of treatment cycles per patient was not available because of variability between the units used per patient. The number of treatment cycles was therefore used as an alternative, which is generally an accepted pharmaco-epidemiological approach [91] and, in our experience, one that is accepted by health authorities when companies submit product-specific Periodic Benefit Risk Evaluation Reports. In the systematic review of clinical studies, the number of patients treated was available, however, not presented to keep the consistency in reporting between the literature review and the review of Global Safety Database of Merck KGaA, Darmstadt, Germany. In the systematic review, the publications included a mixture of study designs, which included different durations of follow-up and monitoring for ADRs. There may be the potential for under-reporting, as most of the studies were powered for efficacy rather than safety, and sometimes safety events are combined or not reported in primary publications. The systematic review only included studies which stated the starting dose and where this dose was within the range stated in the prescribing information; this resulted in the inclusion of a greater number of RCTs than studies with other designs. The severity of OHSS was based upon the diagnosis in the publication and there was heterogeneity in the definitions used.
Conclusion
GONAL-f, marketed as GONAL-f RFF in the USA and GONAL-f in rest of the world, has been used for more than 20 years, with low reporting rates of OHSS [ca. 0.007% in the analysis of the Global Safety Database of Merck KGaA, Darmstadt, Germany, and a frequency of 5.19% (severe OHSS with a frequency of 0.19%) in the systematic review of the scientific literature] and thromboembolism (0.0005% in the Global Safety Database of Merck KGaA, Darmstadt, Germany analysis and 0.0% in the systematic review of the scientific literature). Of the 1110 OHSS cases reported in the Global Safety Database of Merck KGaA, Darmstadt, Germany, 130 (11.7%) were classified as severe; of the 272 OHSS cases retrieved from the systematic review searches, 10 (3.7%) were classified as severe, suggesting, unsurprisingly, a tendency for selectively reporting cases of severe OHSS in the Global Safety Database of Merck KGaA, Darmstadt, Germany. Overall, adverse effects from the use of GONAL-f are infrequent and reported rarely, though there is a need for consistent reporting in order to evaluate the true impact of this treatment.
Change history
19 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12325-021-01684-z
References
Merck KGaA. GONAL-f 450 IU (33 mcg) pen. 2018. https://www.medicines.org.uk/emc/medicine/14384. Accessed 2019.
European Medicines Agency. Ovaleap. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/ovaleap. Accessed 2019
European Medicines Agency. Bemfola. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/bemfola. Accessed 2019
Magnusson A, Kallen K, Thurin-Kjellberg A, Bergh C. The number of oocytes retrieved during IVF: a balance between efficacy and safety. Hum Reprod. 2018;33(1):58–64.
Vloeberghs V, Peeraer K, Pexsters A, D'Hooghe T. Ovarian hyperstimulation syndrome and complications of ART. Best Pract Res Clin Obstet Gynaecol. 2009;23(5):691–709.
Mor YS, Schenker JG. Ovarian hyperstimulation syndrome and thrombotic events. Am J Reprod Immunol. 2014;72(6):541–8.
La Marca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Human Reprod Update. 2014;20(1):124–40.
Lunenfeld B, Bilger W, Longobardi S, Kirsten J, D'Hooghe T, Sunkara SK. Decision points for individualized hormonal stimulation with recombinant gonadotropins for treatment of women with infertility. Gynecol Endocrinol. 2019;35(12):1027–36.
Magnusson Å, Nilsson L, Oleröd G, Thurin-Kjellberg A, Bergh C. The addition of anti-Müllerian hormone in an algorithm for individualized hormone dosage did not improve the prediction of ovarian response-a randomized, controlled trial. Hum Reprod. 2017;32(4):811–9.
Oudshoorn SC, van Tilborg TC, Eijkemans MJC, et al. Individualized versus standard FSH dosing in women starting IVF/ICSI: an RCT. Part 2: The predicted hyper responder. Hum Reprod. 2017;32(12):2506–14.
Popovic-Todorovic B, Loft A, Bredkjaeer HE, Bangsbøll S, Nielsen IK, Andersen AN. A prospective randomized clinical trial comparing an individual dose of recombinant FSH based on predictive factors versus a 'standard' dose of 150 IU/day in 'standard' patients undergoing IVF/ICSI treatment. Hum Reprod. 2003;18(11):2275–82.
Griesinger G, Verweij PJ, Gates D, et al. Prediction of ovarian hyperstimulation syndrome in patients treated with Corifollitropin alfa or rFSH in a GnRH antagonist protocol. PLoS One. 2016;11(3):e0149615.
The ESHRE Guideline Group On Ovarian Stimulation, Bosch E, Broer S, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI. Human Reprod Open. 2020;2020(2):hoaa009.
Witz CA, Daftary GS, Doody KJ, et al. Randomized, assessor-blinded trial comparing highly purified human menotropin and recombinant follicle-stimulating hormone in high responders undergoing intracytoplasmic sperm injection. Fertil Steril. 2020;114(2):321–30.
Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393–406.
La Marca A, Papaleo E, Grisendi V, Argento C, Giulini S, Volpe A. Development of a nomogram based on markers of ovarian reserve for the individualisation of the follicle-stimulating hormone starting dose in in vitro fertilisation cycles. BJOG. 2012;119(10):1171–9.
Filipovic-Pierucci A, Gabet A, Deneux-Tharaux C, Plu-Bureau G, Olie V. Arterial and venous complications after fertility treatment: a French nationwide cohort study. Eur J Obstet Gynecol Reprod Biol. 2019;237:57–63.
Wang R, Lin S, Wang Y, Qian W, Zhou L. Comparisons of GnRH antagonist protocol versus GnRH agonist long protocol in patients with normal ovarian reserve: a systematic review and meta-analysis. PLoS One. 2017;12(4):e0175985.
European Medicines Agency. GONAL-f. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/gonal-f. Accessed 2019.
Medical Dictionary for Regulatory Activities. (MedDRA). 2017. https://www.meddra.org/. Accessed 2019.
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Aboulghar MA, Mansour RT, Serour GI, Amin YM, Sattar MA, elAttar E. Recombinant follicle-stimulating hormone in the treatment of patients with history of severe ovarian hyperstimulation syndrome. Fertil Steril. 1996;66(5):757–60.
Arce JC, Klein BM, La Marca A. The rate of high ovarian response in women identified at risk by a high serum AMH level is influenced by the type of gonadotropin. Gynecol Endocrinol. 2014;30(6):444–50.
Shoham Z, Smith H, Yeko T, O'Brien F, Hemsey G, O'Dea L. Recombinant LH (lutropin alfa) for the treatment of hypogonadotrophic women with profound LH deficiency: a randomized, double-blind, placebo-controlled, proof-of-efficacy study. Clin Endocrinol (Oxf). 2008;69(3):471–8.
Sohrabvand F, Sheikhhassani S, Bagheri M, et al. Comparison of highly purified urinary versus recombinant FSH: effect on ART outcomes in polycystic ovary syndrome. Iran J Reprod Med. 2012;10(3):229–36.
Szilagyi A, Bartfai G, Manfai A, Koloszar S, Pal A, Szabo I. Low-dose ovulation induction with urinary gonadotropins or recombinant follicle stimulating hormone in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2004;18(1):17–22.
Yong PY, Brett S, Baird DT, Thong KJ. A prospective randomized clinical trial comparing 150 IU and 225 IU of recombinant follicle-stimulating hormone (Gonal-F*) in a fixed-dose regimen for controlled ovarian stimulation in in vitro fertilization treatment. Fertil Steril. 2003;79(2):308–15.
Altay MGO, Kocak M, Demir B, Beydilli G, Haberal A. Comparison of a novel minimal stimulation protocol with clomiphene citrate plus recombinant follicle-stimulating hormone to recombinant follicle-stimulating hormone alone for ovulation induction: a prospective study. J Turk German Gynecol Assoc. 2007;8(4):397–401.
Arruda JTAM, Maia MCS, da Silva TM, et al. Comparison GnRH agonist short protocol and GnRH antagonist in Brazilian normoresponder patients undergoing their first cycle of controlled ovarian stimulation. J Bras Reprod Assist. 2013;17(5):304–9.
European and Israeli Study Group on Highly Purified Menotropin versus Recombinant Follicle-Stimulating Hormone. Efficacy and safety of highly purified menotropin versus recombinant follicle-stimulating hormone in in vitro fertilization/intracytoplasmic sperm injection cycles: a randomized, comparative trial. Fertil Steril. 2002;78(3):520–8.
Franco JG Jr, Baruffi RL, Coelho J, Mauri AL, Petersen CG, Garbellini E. A prospective and randomized study of ovarian stimulation for ICSI with recombinant FSH versus highly purified urinary FSH. Gynecol Endocrinol. 2000;14(1):5–10.
Lenton E, Soltan A, Hewitt J, et al. Induction of ovulation in women undergoing assisted reproductive techniques: recombinant human FSH (follitropin alpha) versus highly purified urinary FSH (urofollitropin HP). Hum Reprod. 2000;15(5):1021–7.
Nyboe Andersen A, Nelson SM, Fauser BC, Garcia-Velasco JA, Klein BM, Arce JC. Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril. 2017;107(2):387–96.e4.
Rettenbacher M, Andersen AN, Garcia-Velasco JA, et al. A multi-centre phase 3 study comparing efficacy and safety of Bemfola® versus Gonal-f® in women undergoing ovarian stimulation for IVF. Reprod Biomed Online. 2015;30(5):504–13.
Bergh C, Howles CM, Borg K, et al. Recombinant human follicle stimulating hormone (r-hFSH; Gonal-F) versus highly purified urinary FSH (Metrodin HP): results of a randomized comparative study in women undergoing assisted reproductive techniques. Hum Reprod. 1997;12(10):2133–9.
Brinsden P, Akagbosu F, Gibbons LM, et al. A comparison of the efficacy and tolerability of two recombinant human follicle-stimulating hormone preparations in patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2000;73(1):114–6.
Casadei L, Puca F, Emidi E, Manicuti C, Madrigale A, Piccione E. Sequential low-dose step-up and step-down protocols with recombinant follicle-stimulating hormone in polycystic ovary syndrome: prospective comparison with step-down protocol. Arch Gynecol Obstet. 2012;286(5):1291–7.
Caserta D, Lisi F, Marci R, et al. Does supplementation with recombinant luteinizing hormone prevent ovarian hyperstimulation syndrome in down regulated patients undergoing recombinant follicle stimulating hormone multiple follicular stimulation for IVF/ET and reduces cancellation rate for high risk of hyperstimulation? Gynecol Endocrinol. 2011;27(11):862–6.
Doldi N, Persico P, Di Sebastiano F, Marsiglio E, Ferrari A. Gonadotropin-releasing hormone antagonist and metformin for treatment of polycystic ovary syndrome patients undergoing in vitro fertilization-embryo transfer. Gynecol Endocrinol. 2006;22(5):235–8.
Filicori M, Cognigni GE, Pocognoli P, et al. Comparison of controlled ovarian stimulation with human menopausal gonadotropin or recombinant follicle-stimulating hormone. Fertil Steril. 2003;80(2):390–7.
Frydman R, Howles CM, Truong F. A double-blind, randomized study to compare recombinant human follicle stimulating hormone (FSH; Gonal-F) with highly purified urinary FSH (Metrodin) HP) in women undergoing assisted reproductive techniques including intracytoplasmic sperm injection. The French Multicentre Trialists. Hum Reprod. 2000;15(3):520–5.
Hedon B, Hugues JN, Emperaire JC, et al. A comparative prospective study of a chronic low dose versus a conventional ovulation stimulation regimen using recombinant human follicle stimulating hormone in anovulatory infertile women. Hum Reprod. 1998;13(1O):2688–92.
International Recombinant Human Chorionic Gonadotropin Study Group. Induction of ovulation in World Health Organization group II anovulatory women undergoing follicular stimulation with recombinant human follicle-stimulating hormone: a comparison of recombinant human chorionic gonadotropin (rhCG) and urinary hCG. Fertil Steril. 2001;75(6):1111–8.
Lin YH, Wen YR, Chang Y, et al. Safety and efficacy of mixing cetrorelix with follitropin alfa: a randomized study. Fertil Steril. 2010;94(1):179–83.
Recombinant Human FSH Study Group. Clinical assessment of recombinant human follicle-stimulating hormone in stimulating ovarian follicular development before in vitro fertilization. Fertil Steril. 1995;63(1):77–86.
Moon SY, Choi YS, Ku SY, et al. Comparison of the efficacy and safety of a new recombinant human follicle-stimulating hormone (DA-3801) with follitropin-alpha (Gonal-F) in women undergoing controlled ovarian hyperstimulation for assisted reproductive technology. J Obstet Gynaecol Res. 2007;33(3):305–15.
O'Dea L, O'Brien F, Currie K, Hemsey G. Follicular development induced by recombinant luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in anovulatory women with LH and FSH deficiency: evidence of a threshold effect. Curr Med Res Opin. 2008;24(10):2785–93.
Olivennes F, Howles CM, Borini A, et al. Individualizing FSH dose for assisted reproduction using a novel algorithm: the CONSORT study. Reprod Biomed Online. 2009;18(2):195–204.
Olivennes F, Trew G, Borini A, et al. Randomized, controlled, open-label, non-inferiority study of the CONSORT algorithm for individualized dosing of follitropin alfa. Reprod Biomed Online. 2015;30(3):248–57.
Pierson RA, Olatunbosun OA, Chizen DR, Saunders H, Loumaye E, De Moustier B. Recombinant human luteinizing hormone to trigger ovulation: randomized, controlled, dose-finding pilot study in ovulation induction. J Reprod Med. 2014;59(7–8):355–66.
Rashidi M, Aaleyasin A, Aghahosseini M, Loloi S, Kokab A, Najmi Z. Advantages of recombinant follicle-stimulating hormone over human menopausal gonadotropin for ovarian stimulation in intrauterine insemination: a randomized clinical trial in unexplained infertility. Eur J Obstet Gynecol Reprod Biol. 2013;169(2):244–7.
Schats R, Sutter PD, Bassil S, Kremer JA, Tournaye H, Donnez J. Ovarian stimulation during assisted reproduction treatment: a comparison of recombinant and highly purified urinary human FSH. On behalf of The Feronia and Apis study group. Hum Reprod. 2000;15(8):1691–7.
Scholtes MC, Schnittert B, van Hoogstraten D, Verhoeven HC, Zrener A, Warne DW. A comparison of 3-day and daily follicle-stimulating hormone injections on stimulation days 1–6 in women undergoing controlled ovarian hyperstimulation. Fertil Steril. 2004;81(4):996–1001.
Serour GI, Aboulghar M, Al Bahar A, Hugues JN, Esmat K. Phase IV, open-label, randomized study of low-dose recombinant human follicle-stimulating hormone protocols for ovulation induction. Reprod Biol Endocrinol. 2014;12:52.
Strowitzki T, Kuczynski W, Mueller A, Bias P. Safety and efficacy of Ovaleap® (recombinant human follicle-stimulating hormone) for up to 3 cycles in infertile women using assisted reproductive technology: a phase 3 open-label follow-up to Main Study. Reprod Biol Endocrinol. 2016;14(1):31.
Taketani Y, Kelly E, Yoshimura Y, et al. Recombinant follicle-stimulating hormone (follitropin alfa) for ovulation induction in Japanese patients with anti-estrogen-ineffective oligo- or anovulatory infertility: results of a phase II dose-response study. Reprod Med Biol. 2010;9(2):91–7.
Taketani Y, Kelly E, Yoshimura Y, et al. Recombinant follicle-stimulating hormone (follitropin alfa) versus purified urinary follicle-stimulating hormone in a low-dose step-up regimen to induce ovulation in Japanese women with anti-estrogen-ineffective oligo- or anovulatory infertility: results of a single-blind phase III study. Reprod Med Biol. 2010;9(2):99–106.
Figen Turkcapar A, Seckin B, Onalan G, Ozdener T, Batioglu S. Human menopausal gonadotropin versus recombinant FSH in polycystic ovary syndrome patients undergoing in vitro fertilization. Int J Fertil Steril. 2013;6(4):238–43.
Weigert M, Krischker U, Pohl M, Poschalko G, Kindermann C, Feichtinger W. Comparison of stimulation with clomiphene citrate in combination with recombinant follicle-stimulating hormone and recombinant luteinizing hormone to stimulation with a gonadotropin-releasing hormone agonist protocol: a prospective, randomized study. Fertil Steril. 2002;78(1):34–9.
Prajapati K, Desai M, Shah S, Choudhary S, Aggarwal R, Mishra V. Treatment outcome of ovulation-inducing agents in patients with anovulatory infertility: a prospective, observational study. J Pharmacol Pharmacother. 2017;8(3):116–21.
Behrouzi Lak T, Hajshafiha M, Nanbakhsh F, Oshnouei S. N-Acetyl cysteine in ovulation induction of PCOS women underwent intrauterine insemination: an RCT. Int J Reprod Biomed. 2017;15(4):203–8.
Heiser PWFR, Seifu Y, Yankov V, Daftary GS. Highly purified human menotropin (HPhMG) is associated with a low incidence of ovarian hyperstimulation syndrome (OHSS) in patients undergoing in vitro fertilization: Menopur in GnRH antagonist single embryo transfer - High Responder (MEGASET-HR) trial outcomes. Fertil Steril. 2018;3:e30–e3131.
Hussain M, Cahill D, Akande V, Gordon U. Discrepancies between antimullerian hormone and follicle stimulating hormone in assisted reproduction. Obstet Gynecol Int. 2013;2013:383278.
Ersahin AA, Arpaci H, Ersahin SS, Celik N, Acet M. AFC vs. AMH: prediction of ovarian response in women with endometrioma undergoing controlled ovarian stimulation. Eur Rev Med Pharmacol Sci. 2017;21(10):2499–503.
Ebrahimi M, Akbari-Asbagh F, Ghalandar-Attar M. Letrozole+ GnRH antagonist stimulation protocol in poor ovarian responders undergoing intracytoplasmic sperm injection cycles: an RCT. Int J Reprod Biomed (Yazd). 2017;15(2):101–8.
Lu HF, Peng FS, Chen SU, Chiu BC, Yeh SH, Hsiao SM. A preliminary report of a low-dose step-up regimen of recombinant human FSH for young women undergoing ovulation induction with IUI. Int J Fertil Steril. 2016;9(4):436–41.
European Medicines Agency. Rekovelle: follitropin delta. 2017. https://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003994/human_med_002044.jsp&mid=WC0b01ac058001d124. Accessed 2019.
European Medicines Agency. Puregon. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/puregon. Accessed 2019
Mastrangeli R, Satwekar A, Cutillo F, Ciampolillo C, Palinsky W, Longobardi S. In-vivo biological activity and glycosylation analysis of a biosimilar recombinant human follicle-stimulating hormone product (Bemfola) compared with its reference medicinal product (GONAL-f). PLoS One. 2017;12(9):e0184139.
Trew GH, Brown AP, Gillard S, et al. In vitro fertilisation with recombinant follicle stimulating hormone requires less IU usage compared with highly purified human menopausal gonadotrophin: results from a European retrospective observational chart review. Reprod Biol Endocrinol. 2010;8:137.
Lensen SF, Wilkinson J, Leijdekkers JA, et al. Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI). Cochrane Database Syst Rev. 2018;2:CD012693.
Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update. 2002;8(6):559–77.
Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv. 1989;44(6):430–40.
Schenker JG, Ezra Y. Complications of assisted reproductive techniques. Fertil Steril. 1994;61(3):411–22.
Abramov Y, Elchalal U, Schenker JG. Severe OHSS: an 'epidemic' of severe OHSS: a price we have to pay? Hum Reprod. 1999;14(9):2181–3.
Smitz J, Camus M, Devroey P, Erard P, Wisanto A, Van Steirteghem AC. Incidence of severe ovarian hyperstimulation syndrome after GnRH agonist/hMG superovulation for in-vitro fertilization. Hum Reprod. 1990;5(8):933–7.
Asch RH, Li HP, Balmaceda JP, Weckstein LN, Stone SC. Severe ovarian hyperstimulation syndrome in assisted reproductive technology: definition of high risk groups. Hum Reprod. 1991;6(10):1395–9.
Brinsden PR, Wada I, Tan SL, Balen A, Jacobs HS. Diagnosis, prevention and management of ovarian hyperstimulation syndrome. Br J Obstet Gynaecol. 1995;102(10):767–72.
Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2016;4:Cd001750.
Dosouto C, Haahr T, Humaidan P. Gonadotropin-releasing hormone agonist (GnRHa) trigger—state of the art. Reprod Biol. 2017;17(1):1–8.
Humaidan P, Nelson SM, Devroey P, et al. Ovarian hyperstimulation syndrome: review and new classification criteria for reporting in clinical trials. Hum Reprod. 2016;31(9):1997–2004.
Practice Committee of the American Society for Reproductive Medicine. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. 2016;106(7):1634–47.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–96.
Huang Y-L, Moon J, Segal JB. A comparison of active adverse event surveillance systems worldwide. Drug Saf. 2014;37(8):581–96.
Hershkop E, Segal L, Fainaru O, Kol S. 'Model' versus 'everyday' patients: can randomized controlled trial data really be applied to the clinic? Reprod Biomed Online. 2017;34(3):274–9.
Lineberry N, Berlin JA, Mansi B, et al. Recommendations to improve adverse event reporting in clinical trial publications: a joint pharmaceutical industry/journal editor perspective. BMJ. 2016;355:i5078.
Joint Society of Obstetricians and Gynaecologists of Canada-Canadian Fertility and Andrology Society Clinical Practice Guidelines Committee, Shmorgun D, Claman P, et al. The diagnosis and management of ovarian hyperstimulation syndrome: No. 268, November 2011. Int J Gynaecol Obstet. 2012;116(3):268–73.
Smith V, Osianlis T, Vollenhoven B. Prevention of ovarian hyperstimulation syndrome: a review. Obstet Gynecol Int. 2015;2015:514159.
Lee TH, Liu CH, Huang CC, et al. Serum anti-Mullerian hormone and estradiol levels as predictors of ovarian hyperstimulation syndrome in assisted reproduction technology cycles. Hum Reprod. 2008;23(1):160–7.
La Marca A, Blockeel C, Bosch E, et al. Individualized FSH dosing improves safety and reduces iatrogenic poor response while maintaining live-birth rates. Hum Reprod. 2018;33(5):982–3.
Strom BL. Pharmacoepidemiology. 4th ed. Chichester: Wiley; 2005. p. 176.
Acknowledgements
Funding
This study and the journal’s Rapid Service and Open Access Fees were funded by Merck KGaA, Darmstadt, Germany.
Medical Writing and Other Assistance
The authors would like to thank Alexander Jones, Diana Marouco, Evelina Matekonyte and Steven Goodrick of inScience Communications, Springer Healthcare Ltd, UK, for providing literature research and medical writing support in the preparation of this manuscript. This support was funded by Merck KGaA, Darmstadt, Germany.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors contributed to the conception and design of the analysis, as well as interpretation of data and critical review of this manuscript. All authors approved the manuscript for submission to the journal.
Prior Presentation
These data were presented at ESHRE 2019, Vienna, Austria.
Disclosures
Erica Velthuis and Thomas D’Hooghe are employees of Merck KGaA, Darmstadt, Germany. Salvatore Longobardi is an employee of Merck Serono SpA, Rome, Italy, an affiliate of Merck KGaA, Darmstadt, Germany. Julie Hubbard is an employee of EMD Serono Research & Development Institute, Billerica, MA, USA, a business of Merck KGaA, Darmstadt, Germany.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The primary outcome data obtained from the literature searches are available from the published papers. The data from the Global Safety Database of Merck KGaA, Darmstadt, Germany are not publicly available, owing to commercial reasons, but are reported to health authorities according to good pharmacovigilance practices.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12967055.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Velthuis, E., Hubbard, J., Longobardi, S. et al. The Frequency of Ovarian Hyperstimulation Syndrome and Thromboembolism with Originator Recombinant Human Follitropin Alfa (GONAL-f) for Medically Assisted Reproduction: A Systematic Review. Adv Ther 37, 4831–4847 (2020). https://doi.org/10.1007/s12325-020-01512-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01512-w