Abstract
Purpose
To determine the optimal regimen of recombinant human follicle-stimulating hormone (r-hFSH) for ovulation induction (OI) in Japanese women with amenorrhea I or anovulatory infertility.
Methods
In this randomized, double-blind, dose-finding study, women aged 20–39 years were enrolled. Patients underwent a chronic low-dose step-up regimen with starting doses of r-hFSH of 37.5 IU (group L; n = 62), 75 IU (group M; n = 62) or 150 IU (group H; n = 60). Primary endpoint was time to achieve a dominant follicle with mean diameter ≥18 mm. Secondary endpoints included percentage of patients producing a dominant follicle ≥18 mm, achieving ovulation, and biochemical pregnancy.
Results
Median time to achieve a dominant follicle ≥18 mm was 14 days (group L; 87.7%), 10 days (group M; 98.4%), and 8 days (group H; 94.5%). In group M, ovulation occurred in 95.1% of patients, with pregnancy in 18.0%, compared with 86.0% and 15.8% in group L, and 50.9% and 9.1% in group H, respectively. Twelve patients developed ovarian hyperstimulation syndrome (nine in group H). Most adverse events in groups L and M were mild in severity.
Conclusions
A starting dose of 75 IU r-hFSH was associated with a favorable efficacy and safety profile for OI in Japanese women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recombinant human follicle-stimulating hormone (r-hFSH) is widely used for ovulation induction (OI) in women with oligo- or anovulatory infertility. The amount of exogenous FSH required to induce follicle development (the so-called FSH threshold) is, however, highly variable among different women [1, 2]. Although Japanese terminology relating to oligo- or anovulatory infertility differs from standard World Health Organization (WHO) nomenclature, Japanese patients with “amenorrhea I or anovulatory cycles” are similar to the population with WHO group II oligo- or anovulatory infertility: both groups of patients are defined by disordered ovulation, a positive response to a progestin challenge (suggesting continued estrogen production), and the inclusion of a subset of patients with polycystic ovary syndrome (PCOS). Indeed, most patients with anovulatory infertility relating to hypothalamic–pituitary dysfunction have PCOS [3].
The ovaries of women with PCOS are extremely sensitive to gonadotropin stimulation, with the result that mono-ovulation is particularly difficult to achieve [4]. Importantly, development of multiple dominant follicles carries risks of ovarian hyperstimulation syndrome (OHSS) and multiple pregnancy [5–8]. The chronic low-dose step-up r-hFSH regimen is designed to attain and maintain monofollicular development (i.e., development of a single dominant follicle) [9]. A frequently used protocol involves a daily dose of 75 IU of r-hFSH for 14 days, followed by small, incremental dose increases (of 37.5 IU) at intervals of at least 7 days [4, 10]. Variations in response to therapies are, however, known to occur between different ethnic groups, including Asian patients, and the prescribed daily dose of a number of drugs is lower in Japan than in the USA or Europe [11]. The pharmacokinetic profile of r-hFSH in Japanese individuals is similar to that in other populations (unpublished data), so standard doses of r-hFSH may be appropriate for Japanese patients undergoing OI.
The present phase II study investigated the efficacy and safety of r-hFSH for OI in Japanese women with amenorrhea I or anovulatory cycles, and determined the optimal starting dose and dosing regimen of r-hFSH in these patients.
Materials and methods
Study design
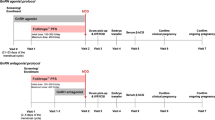
This was a phase II, randomized, double-blind, parallel-group, dose–response study of r-hFSH (follitropin alfa; GONALEF®/GONAL-f®; Merck Serono S.A.-Geneva, Switzerland). The trial was designed to assess the duration of r-hFSH dosing required to achieve a dominant follicle of ≥18 mm.
The study was conducted by Merck Serono Co., Ltd., Japan between 2001 and 2003, at 22 medical institutions in Japan (protocol number 22377). The institutional review boards of all participating investigational centers approved the study for their respective center, and written informed consent was obtained from each participant. The study was conducted in accordance with good clinical practice and the Declaration of Helsinki (October 2000).
Patients
Infertile women aged 20–39 years with body mass index (BMI) of 17–28 kg/m2 were eligible for enrollment. Patients with anovulation caused by hypothalamic or pituitary dysfunction (with or without PCOS) were enrolled based on Japanese national diagnostic criteria of amenorrhea I [positive progesterone (P4) withdrawal test] or anovulatory cycles (oligo- or polymenorrhea). Women who had failed to achieve a pregnancy after at least two cycles of antiestrogen therapy (including clomiphene citrate or cyclofenil) were eligible for treatment. Key exclusion criteria included presence of sex-hormone-dependent tumors, ovarian enlargement not due to PCOS, unexplained genital bleeding, and moderate or severe dysfunction of the heart, lungs, liver, kidneys or cardiovascular system. Additional exclusion criteria included baseline serum FSH levels ≥20 mIU/mL or serum prolactin ≥15 ng/mL.
Treatment schedule
Using a central randomization method, patients were allocated blindly to one of three treatment groups (1:1:1) with different starting daily doses of r-hFSH (group L: 37.5 IU; group M: 75 IU; group H: 150 IU). Randomization was stratified by PCOS status, age, and BMI. All patients received r-hFSH (lyophilized powder reconstituted with 1 mL water for injection) administered by subcutaneous injection in a chronic low-dose step-up regimen.
Dosing began 3–7 days after spontaneous or P4-induced withdrawal bleeding, and the allocated starting dose was administered for 7 consecutive days. If the mean diameter of the dominant follicle was <11 mm on day 8 of stimulation, the daily dose of r-hFSH was increased by 37.5 IU; dosing continued without increase if the follicle was ≥11 mm. If the mean diameter of the dominant follicle was still <11 mm on days 15 and 22 of stimulation, two further dosing increments of 37.5 IU were permitted. When the dominant follicle reached a mean diameter of 18 mm, ovulation was triggered using 5000 IU human chorionic gonadotropin (hCG; Gonatrophin® or Pregnyl®; HCG Fuji/Mochida, Tokyo, Japan) administered intramuscularly. Administration of r-hFSH was continued for a maximum of 28 days. hCG administration was permitted if the mean diameter of the dominant follicle reached 18 mm or was ≥16 mm on study day 29, but was cancelled if more than three follicles with a mean diameter of ≥16 mm were visualized or if serum estradiol (E2) levels were >2000 pg/mL.
Ovulation was assumed if serum P4 level was ≥5 ng/mL 8 days after hCG administration or, when serum P4 levels were unavailable, if high basal body temperature persisted for ≥7 days. Patients were followed up for 28 days after administration of hCG, at which point a urinary pregnancy test was performed. The biochemical pregnancy rate was defined as the percentage of women with a positive urine pregnancy test 28 days after r-hFSH administration. Those who did not receive hCG or who menstruated within 28 days of completing r-hFSH were followed up for 14 days after administration of r-hFSH.
Procedures
Transvaginal ultrasonography (TVUS) was performed pre-study (within 28 days before bleeding), on stimulation days 1 (menstrual cycle days 3–7), 8, 15, and 22. In addition, TVUS was performed once or twice per week after each incremental r-hFSH dose increase, as necessary after the dominant follicle reached 16 mm, and 1 and 8 days after cessation of r-hFSH treatment. TVUS was also performed whenever OHSS was suspected.
Endpoints
The primary endpoint of the study was the duration of r-hFSH dosing required to achieve a dominant follicle with a mean diameter of ≥18 mm. Secondary efficacy endpoints were the percentage of patients producing a dominant follicle with a mean diameter ≥18 mm, mean total dose of r-hFSH administered (to patients who achieved a dominant follicle with a mean diameter of ≥18 mm), proportion of patients achieving ovulation (serum P4 level ≥5 ng/mL 8 days after hCG injection), proportion of patients who did not receive hCG (hCG cancellation rate), rate of single-follicle development (defined as the presence of a dominant follicle with a mean diameter ≥18 mm without the presence of other follicles ≥14 mm in diameter), and biochemical pregnancy rate.
Safety assessments included the incidence of adverse events (AEs) and serious AEs (SAEs).
Statistical analysis
The full analysis set (FAS) included all patients who received at least one dose of r-hFSH with no violations of good clinical practice guidelines and no major discrepancies in inclusion or exclusion criteria, and was used for safety evaluation. The per protocol set (PPS) included patients from the FAS who had no serious protocol deviations, and was used as the primary population for efficacy evaluation.
Uniformity across dose groups with regard to baseline characteristics was evaluated using the Kruskal–Wallis test. The log-rank and generalized Wilcoxon tests were used to compare uniformity between groups and assess the dose–response relationship for the primary efficacy endpoint. Kaplan–Meier methods were used to estimate the cumulative ratios of patients who achieved dominant follicular development with a mean diameter of ≥18 mm. The uniformity and dose response of secondary efficacy endpoints (with the exception of pregnancy rate) were analyzed using Fisher’s exact or one-way analysis of variance (ANOVA) tests. Descriptive statistics were used to compare the biochemical pregnancy rates.
Results
Patient disposition
In total, 201 patients were enrolled and randomly assigned to three treatment groups (Fig. 1). The FAS comprised 184 patients from groups L (n = 62), M (n = 62), and H (n = 60). The PPS comprised 173 patients (group L, n = 57; group M, n = 61; group H, n = 55). Overall, 15 patients (group L, n = 3; group M, n = 1; group H, n = 11) discontinued treatment or dropped out of the study.
Exposure
In the PPS population, the mean [standard deviation (SD)] daily dose of r-hFSH administered was 51.0 (16.11) IU in group L, 81.2 (11.73) IU in group M, and 150.1 (0.63) IU in group H. The median daily dose in group L rose to 50 IU, while that of groups M and H remained at 75 and 150 IU, respectively. The proportion of patients who required at least one dose increase was 54.4% (31/57) in group L, 27.9% (17/61) in group M, and 1.8% (1/55) in group H.
Baseline demographic and disease characteristics
At baseline, patients in the FAS had a mean (SD) age of 31.3 (3.93) years and a mean BMI of 21.4 (2.99) kg/m2. No significant differences in clinical characteristics (diagnosis of amenorrhea I, anovulatory cycles, and history of PCOS) were found across treatment groups (Kruskal–Wallis test).
Pre-study levels of FSH, luteinizing hormone, and E2 were similar across treatment groups (data not shown). Mean (SD) prolactin levels (ng/mL) were significantly different in groups L [6.4 (2.64)], M [5.2 (2.54)], and H [5.9 (2.52); P = 0.0287; Kruskal–Wallis test], although no patients had prolactin levels above the normal range at baseline.
Efficacy data
In patients in the PPS, there was a significant dose response in the median time to achieve a dominant follicular diameter of 18 mm, in the order: group L > group M > group H. The median time taken to achieve a dominant follicle ≥18 mm in diameter was 14 days (group L), 10 days (group M), and 8 days (group H) (P < 0.0001; log-rank and generalized Wilcoxon tests; Table 1). No response to r-hFSH was observed in any dose group until after day 4 of treatment. Between-group differences in response to r-hFSH became apparent on day 8 (Fig. 2).
Almost all patients in group M (60/61; 98.4%) achieved a dominant follicle of 18 mm in mean diameter (Table 1), but there was no significant difference between groups for this outcome (P = 0.0617; Fisher’s exact test). The mean (SD) total dose of r-hFSH required to achieve a dominant follicle of ≥18 mm correlated with the dose group (group L < group M < group H), with a significant difference between groups in the mean total dose of r-hFSH administered (P = 0.0006; ANOVA). A similar correlation between the mean (SD) daily dose of r-hFSH required for a dominant follicle ≥18 mm to be achieved and the dose group was observed.
The highest rates of ovulation were observed in group M (58/61; 95.1%), while the hCG cancellation rate was highest in group H (21/55; 38.2%). More than half of the patients in groups L and M achieved monofollicular development, but a smaller proportion of patients achieved development of a single dominant follicle in group H (4/55; 7.3%). For all three outcomes (ovulation rate, hCG cancellation rate, and rate of monofollicular development), there was a significant difference between groups (P < 0.0001; Fisher’s exact test; Table 1).
A total of 25 patients achieved a biochemical pregnancy, and the rate was highest in group M (11/61; 18.0%; Table 1), although no tests of statistical significance were performed. Twenty patients gave birth to 23 babies (group L, n = 7; group M, n = 9; group H, n = 4); three patients (one in each group) delivered twins. No congenital abnormalities were reported in the neonates.
Similar results were obtained in subgroup analyses of patients with and without PCOS; the results were comparable between subgroups for any endpoint.
Safety data
In the FAS population, 113/184 patients (61.4%) experienced a total of 266 AEs; 74 events occurred in 30/62 patients (48.4%) in group L, 101 events in 41/62 patients (66.1%) in group M, and 91 events in 42/60 patients (70.0%) in group H. Most of these AEs were mild (223/266; 83.8%) or moderate (39/266; 14.7%) in severity. The most frequently reported AEs were swollen abdomen, lower abdominal pain, ovarian disorder, ascites, OHSS, headache, and fever (Table 2). Overall, 12 cases of OHSS (≥grade 2 severity) occurred in 12 patients (6.5%); moderate OHSS (grade 2): 1 in group L, 2 in group M, and 8 in group H; severe OHSS (grade 3): 1 in group H. The incidence of OHSS for patients without PCOS (7%) was similar to that in patients with PCOS (6%). Only minor hematological and biochemical abnormalities were reported during the study.
Eight patients experienced eight SAEs and required hospitalization. These comprised three cases of OHSS (one mild, one moderate, and one severe case; all in group H), two missed miscarriages (managed surgically; one each in groups L and M), one intrauterine death (one 8-week-old fetus of a twin pregnancy; group M), one ectopic pregnancy (requiring oophorectomy; group H), and one suspected ectopic pregnancy (managed medically; group M).
In addition, three singleton miscarriages occurred (group L, n = 2; group M, n = 1), miscarriage of one of twins occurred in three pregnancies (one in each group), and one premature birth occurred in group L; it was difficult to assess the relationship of these events with the investigational product, and incidences were not higher relative to the incidences observed in spontaneous pregnancy or pregnancy resulting from infertility therapy.
Discussion
This is the first and possibly the best method to assess the optimal dose regimen of r-hFSH for OI in patients undergoing fertility treatment. In recent years, efforts have been made to reduce the incidence of OHSS and multiple pregnancies associated with OI therapy while simultaneously preserving the efficacy of commonly used regimens [12]. The present study aimed to identify a starting dose of r-hFSH (37.5, 75 or 150 IU) in a step-up regimen by which the threshold for achieving follicle development would be reached quickly and safely. The step-up regimen was an approach employed to achieve ovulation at a serum FSH level slightly above the threshold and, thus, minimize the risk of OHSS and multiple pregnancies [13].
The efficacy of r-hFSH was evaluated using data from 173 patients in the PPS (group L, n = 57; group M, n = 61; group H, n = 55). Overall, there was a significant correlation between the r-hFSH dose group and the duration of dosing required to achieve a dominant follicle of ≥18 mm (primary endpoint), with dominant follicular growth achieved more rapidly in higher r-hFSH dose groups. No response to r-hFSH was observed in any group until after day 4; this is in keeping with the pharmacokinetic profile of r-hFSH, which takes 4 days to reach a steady state after daily subcutaneous administration of a consistent dose [14].
Almost all patients (98.4%) who received a starting r-hFSH dose of 75 IU (group M) produced a dominant follicle with a mean diameter of ≥18 mm. Monofollicular development was achieved in more than half of the patients in groups L and M, whereas few patients developed a single mature follicle in group H. The rate of ovulation in group H was lower than in other groups, reflecting the proportion of patients in this group who met the criteria for hCG (and thus cycle) cancellation. The pregnancy rate in the study was highest in group M (11/61; 18.0%); the low pregnancy rate observed in group H (9.1%) is similarly likely to be the result of cycle cancellation. Twin pregnancies occurred in 3/20 (15.0%) pregnancies in the study, one in each treatment group. The low patient numbers prevent comparisons of multiple pregnancy rates between treatment groups.
Overall, the 37.5 and 75 IU r-hFSH dose groups produced a monofollicular development rate greater than 50% and had few hCG cancellations. Compared with the other two treatment groups, the 75 IU r-hFSH dose group produced the highest proportion of patients achieving a mean dominant follicle diameter of 18 mm and the highest ovulation rate. Furthermore, more than half of the patients in the 37.5 IU group needed at least one dose increase, which prolonged the dosing period and suggests that ovarian stimulation was relatively weak.
There were no unexpected safety findings with low-dose r-hFSH in this Japanese population. In particular, the safety profiles of groups L and M, with starting doses of 37.5 and 75 IU r-hFSH, respectively, were favorable and associated with low rates of OHSS. Unsurprisingly, OHSS (≥grade 2 severity) occurred most frequently in the highest dose group (group H). Overall, these findings are similar to previous published outcomes of r-hFSH (starting dose 75 IU) for OI in other patient populations [4, 15].
In a review of data from 11 published studies employing a chronic low-dose step-up FSH treatment regimen for women with PCOS (starting dose 75 IU; n = 717), Homburg and Howles reported a mono-ovulatory rate of 69% (range 54–88%) and pregnancy rate of 20% per cycle [4]. Importantly, the rates of multiple pregnancy and OHSS were 5.7% (0–14.1%) and 0.14% (range 0–2.4%), respectively. Hugues et al. [15] reported a monofollicular development rate of 54%, a clinical pregnancy rate of 17%, and no cases of severe OHSS among women with WHO group II anovulatory infertility who received a chronic low-dose r-hFSH stimulation protocol (starting dose 75 IU) in a recent French multicenter study.
Baseline characteristics of patients in the current study were similar between groups, except for prolactin levels. Although patients in group L had the highest mean prolactin level, no patient had prolactin levels above the normal range at baseline and, thus, the raised level is unlikely to be of clinical significance.
In summary, results from this phase II dose-finding study show that a starting dose of 75 IU r-hFSH with dose increments of 37.5 IU in a chronic low-dose step-up protocol was associated with the most favorable efficacy and safety profile in Japanese patients with amenorrhea I or anovulatory infertility. The incidence of OHSS was low in the groups with starting doses of 37.5 and 75 IU r-hFSH. However, the regimen with a starting dose of 75 IU r-hFSH was associated with a shorter dosing period and fewer dose increases than the regimen with a starting dose of 37.5 IU r-hFSH. A prospective evaluation of this 75 IU r-hFSH dose has since been performed in a phase III, multicenter, single-blind, parallel-group clinical trial in Japanese patients with anovulatory infertility to confirm its efficacy and safety (unpublished observations).
References
Schoemaker J, van Weissenbruch MM, Scheele F, van der Meer M. The FSH threshold concept in clinical ovulation induction. Baillière’s Clin Obstet Gynaecol. 1993;7:297–308.
Brown JB. Pituitary control of ovarian function—concepts derived from gonadotrophin therapy. Aust N Z J Obstet Gynaecol. 1978;18:46–54.
Broekmans FJ, Knauff EAH, Valkenburg O, Laven JS, Eijkemans MJ, Fauser BCJM. PCOS according to the Rotterdam consensus criteria: change in prevalence among WHO-II anovulation and association with metabolic factors. Br J Obstet Gynaecol. 2006;113:1210–7.
Homburg R, Howles CM. Low-dose FSH therapy for anovulatory infertility associated with polycystic ovary syndrome: rationale, results, reflections and refinements. Hum Reprod Update. 1999;5:493–9.
Gleicher N, Oleske DM, Tur-Kaspa I, Vidali A, Karande V. Reducing the risk of high-order multiple pregnancy after ovarian stimulation with gonadotropins. N Engl J Med. 2000;343:2–7.
Aboulghar MA, Mansour RT. Ovarian hyperstimulation syndrome: classifications and critical analysis of preventive measures. Hum Reprod Update. 2003;9:275–89.
Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update. 2002;8:559–77.
Tummon I, Gavrilova-Jordan L, Allemand MC, Session D. Polycystic ovaries and ovarian hyperstimulation syndrome: a systematic review. Acta Obstet Gynecol Scand. 2005;84:611–6.
Homburg R. Clomiphene citrate—end of an era? A mini-review. Hum Reprod. 2005;20:2043–51.
Homburg R, Insler V. Ovulation induction in perspective. Hum Reprod Update. 2002;8:449–62.
Balant LP, Balant-Gorgia EA. Cultural differences: implications on drug therapy and global drug development. Int J Clin Pharmacol Ther. 2000;38:47–52.
Fauser BC, Van Heusden AM. Manipulation of human ovarian function: physiological concepts and clinical consequences. Endocr Rev. 1997;18:71–106.
Homburg R, Levy T, Ben-Rafael Z. A comparative prospective study of conventional regimen with chronic low-dose administration of follicle-stimulating hormone for anovulation associated with polycystic ovary syndrome. Fertil Steril. 1995;63:729–33.
le Contonnec JY, Porchet HC, Beltrami V, Khan A, Toon S, Rowland M. Clinical pharmacology of recombinant human follicle-stimulating hormone. II. Single doses and steady state pharmacokinetics. Fertil Steril. 1994;61:679–86.
Hugues JN, Cedrin-Durnerin I, Howles CM, Amram M, Angelini A, Balen A, et al. The use of a decremental dose regimen in patients treated with a chronic low-dose step-up protocol for WHO Group II anovulation: a prospective randomized multicentre study. Hum Reprod. 2006;21:2817–22.
Acknowledgments
This study was supported by Merck Serono Co. Ltd., Japan. The authors would like to thank the following investigators: Dr. Kazuo Sengoku, Asahikawa Medical College Hospital; Dr. Shunsaku Fujii, Hirosaki University Hospital; Dr. Jun Fukuda, Akita University Hospital; Dr. Toshifumi Takahashi, Yamagata University Hospital; Dr. Takashi Murakami, Tohoku University Hospital; Dr. Masahiro Ito, Gunma University Hospital; Dr. Naoki Hayashi, Saitama Medical Centre, Saitama Medical College; Dr. Mikio Momoeda, Tokyo University Hospital; Dr. Takashi Okai, Showa University Hospital; Dr. Harumi Kubo, Toho University Ohmori Hospital; Dr. Mario Ikeda, Yokohama City University Medical Centre; Dr. Motomu Andoh, Kyorin University Hospital; Dr. Kazuhiko Hoshi, Yamanashi Medical College Hospital; Dr. Asomi Sato, Kobe University Hospital; Dr. Tasuku Harada, Tottori University Hospital; Dr. Nobuyuki Morioka, Kochi Medical College Hospital; Dr. Ritsuo Honda, Kumamoto University Hospital; Dr. Masato Sanbe, Miyazaki Medical College Hospital; Dr. Tsutomu Dohchi, Kagoshima University Hospital. The authors also thank Hannah Wills and Jane Davies of Caudex Medical (supported by Merck Serono Co., Ltd., Japan) for assistance in the preparation of this manuscript.
Conflict of interest statement
E.K. was previously employed by EMD Serono, Inc. T.Y. acts as a consultant for Merck Serono Co., Ltd., Japan. Z.B. is an employee of Merck Serono S.A.-Geneva, Switzerland. Y.T., Y.Y., H.H., M.I., H.M., and K.A. are employees of individual commercial organizations that provide medical services. H.S. has nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the Japanese r-hFSH Ovulation Induction study group.
EMD Serono, Inc., Merck Serono Co., Ltd., Japan, and Merck Serono S.A.-Geneva, Switzerland are affiliates of Merck KGaA, Darmstadt, Germany.
About this article
Cite this article
Taketani, Y., Kelly, E., Yoshimura, Y. et al. Recombinant follicle-stimulating hormone (follitropin alfa) for ovulation induction in Japanese patients with anti-estrogen-ineffective oligo- or anovulatory infertility: results of a phase II dose–response study. Reprod Med Biol 9, 91–97 (2010). https://doi.org/10.1007/s12522-009-0044-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12522-009-0044-7