Abstract
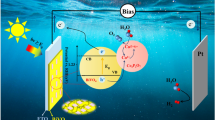
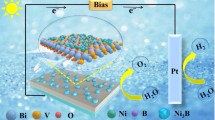
Photoelectrochemical (PEC) water splitting using semiconductors offers a promising way to convert renewable solar energy to clean hydrogen fuels. However, due to the sluggish reaction kinetics of water oxidation, significant charge recombination occurred at the photoanode/electrolyte interface and cause decrease of its PEC performance. To reduce the surface recombination, we deposit different transition metal complexes on BiVO4 nanocone arrays by a versatile light driven in-situ two electrode photodeposition approach without applied bias. Conformal cobalt phosphate “Co-Pi”, nickel borate “Ni-Bi” and manganese phosphate “Mn-Pi” complexes were deposited on BiVO4 nanocone arrays to form core-shell structure photoanode, all of which lead to enhanced photoelectrochemical performance. The photocurrent of the Co-Pi/BiVO4 photoanode under front-side illumination for 5 min is increased by 4 folds comparing to that of bare BiVO4 photoanode at 0.6 V vs. RHE, reaching a hole transfer efficiency as high as 94.5% at 1.23 V vs. RHE. The proposed photodeposition strategy is simple and efficient, and can be extended to deposite cocatalyst on other semiconductors with a valence band edge located at a potential more positive than the oxidation potential of transition metal ion in the cocatalyst.

Similar content being viewed by others
References
Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature1972, 238, 37–38.
Grätzel, M. Photoelectrochemical cells. Nature2001, 414, 338–344.
Tachibana, Y.; Vayssieres, L.; Durrant, J. R. Artificial photosynthesis for solar water-splitting. Nat. Photonics2012, 6, 511–518.
Guo, L. J.; Chen, Y. B.; Su, J. Z.; Liu, M. C.; Liu, Y. Obstacles of solar-powered photocatalytic water splitting for hydrogen production: A perspective from energy flow and mass flow. Energy2019, 172, 1079–1086.
Osterloh, F. E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev.2013, 42, 2294–2320.
Gür, T. M.; Bent, S. F.; Prinz, F. B. Nanostructuring materials for solar-to-hydrogen conversion. J. Phys. Chem. C2014, 118, 21301–21315.
Sivula, K.; van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater.2016, 1, 15010.
Su, J. Z.; Vayssieres, L. A place in the sun for artificial photosynthesis? ACS Energy Lett.2016, 1, 121–135.
Tokunaga, S.; Kato, H.; Kudo, A. Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem. Mater.2001, 13, 4624–4628.
Kim, T. W.; Choi, K. S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science2014, 343, 990–994.
Jo, W. J.; Kang, H. J.; Kong, K. J.; Lee, Y. S.; Park, H.; Lee, Y.; Buonassisi, T.; Gleason, K. K.; Lee, J. S. Phase transition-induced band edge engineering of BiVO4 to split pure water under visible light. Proc. Natl. Acad. Sci. USA2015, 112, 13774–13778.
Park, Y.; McDonald, K. J.; Choi, K. S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev.2013, 42, 2321–2337.
Gao, B.; Wang, T.; Fan, X. L.; Gong, H.; Meng, X. G.; Li, P.; Feng, Y. Y.; Huang, X. L.; He, J. P.; Ye, J. H. Selective deposition of Ag3PO4 on specific facet of BiVO4 nanoplate for enhanced photoelectrochemical performance. Sol. RRL2018, 2, 1800102.
Abdi, F. F.; Savenije, T. J.; May, M. M.; Dam, B.; van de Krol, R. The origin of slow carrier transport in BiVO4 thin film photoanodes: A time-resolved microwave conductivity study. J. Phys. Chem. Lett.2013, 4, 2752–2757.
Chhetri, M.; Dey, S.; Rao, C. N. R. Photoelectrochemical oxygen evolution reaction activity of amorphous Co–La double hydroxide-BiVO4 fabricated by pulse plating electrodeposition. ACS Energy Lett.2017, 2, 1062–1069.
Zachäus, C.; Abdi, F. F.; Peter, L. M.; van de Krol, R. Photocurrent of BiVO4 is limited by surface recombination, not surface catalysis. Chem. Sci.2017, 8, 3712–3719.
Ding, C. M.; Shi, J. Y.; Wang, D. G.; Wang, Z. J.; Wang, N.; Liu, G. J.; Xiong, F. Q.; Li, C. Visible light driven overall water splitting using cocatalyst/BiVO4 photoanode with minimized bias. Phys. Chem. Chem. Phys.2013, 15, 4589–4595.
Kanan, M. W.; Nocera, D. G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science2008, 321, 1072–1075.
Roger, I.; Shipman, M. A.; Symes, M. D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem.2017, 1, 0003.
Inoue, Y. Photocatalytic water splitting by RuO2-loaded metal oxides and nitrides with d0- and d10-related electronic configurations. Energy Environ. Sci.2009, 2, 364–386.
Sanchez Casalongue, H. G.; Ng, M. L.; Kaya, S.; Friebel, D.; Ogasawara, H.; Nilsson, A. In situ observation of surface species on iridium oxide nanoparticles during the oxygen evolution reaction. Angew. Chem., Int. Ed.2014, 53, 7169–7172.
Zhang, B.; Zheng, X. L.; Voznyy, O.; Comin, R.; Bajdich, M.; García-Melchor, M.; Han, L. L.; Xu, J. X.; Liu, M.; Zheng, L. R. et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science2016, 352, 333–337.
Liu, R.; Lin, Y. J.; Chou, L. Y.; Sheehan, S. W.; He, W. S.; Zhang, F.; Hou, H. J. M.; Wang, D. W. Water splitting by tungsten oxide prepared by atomic layer deposition and decorated with an oxygen-evolving catalyst. Angew. Chem., Int. Ed.2011, 50, 499–502.
Carroll, G. M.; Zhong, D. K.; Gamelin, D. R. Mechanistic insights into solar water oxidation by cobalt-phosphate-modified α-Fe2O3 photoanodes. Energy Environ. Sci.2015, 8, 577–584.
Steinmiller, E. M. P.; Choi, K. S. Photochemical deposition of cobaltbased oxygen evolving catalyst on a semiconductor photoanode for solar oxygen production. Proc. Natl. Acad. Sci. USA2009, 106, 20633–20636.
Gao, B.; Wang, T.; Fan, X. L.; Gong, H.; Li, P.; Feng, Y. Y.; Huang, X. L.; He, J. P.; Ye, J. H. Enhanced water oxidation reaction kinetics on a BiVO4 photoanode by surface modification with Ni4O4 cubane. J. Mater. Chem. A2019, 7, 278–288.
Pilli, S. K.; Furtak, T. E.; Brown, L. D.; Deutsch, T. G.; Turner, J. A.; Herring, A. M. Cobalt-phosphate (Co-Pi) catalyst modified Mo-doped BiVO4 photoelectrodes for solar water oxidation. Energy Environ. Sci.2011, 4, 5028–5034.
Zhong, D. K.; Choi, S.; Gamelin, D. R. Near-complete suppression of surface recombination in solar photoelectrolysis by “Co-Pi” catalyst-modified W: BiVO4. J. Am. Chem. Soc.2011, 133, 18370–18377.
Wei, Y. K.; Su, J. Z.; Wan, X. K.; Guo, L. J.; Vayssieres, L. Spontaneous photoelectric field-enhancement effect prompts the low cost hierarchical growth of highly ordered heteronanostructures for solar water splitting. Nano Res.2016, 9, 1561–1569.
Wang, L.; Su, J. Z.; Guo, L. J. Hierarchical growth of a novel Mn-Bi coupled BiVO4 arrays for enhanced photoelectrochemical water splitting. Nano Res.2019, 12, 575–580.
Su, J. Z.; Guo, L. J.; Yoriya, S.; Grimes, C. A. Aqueous growth of pyramidal-shaped BiVO4 nanowire arrays and structural characterization: Application to photoelectrochemical water splitting. Cryst. Growth Des.2010, 10, 856–861.
Choi, S. K.; Choi, W.; Park, H. Solar water oxidation using nickelborate coupled BiVO4 photoelectrodes. Phys. Chem. Chem. Phys.2013, 15, 6499–6507.
Joya, K. S.; Joya, Y. F.; De Groot, H. J. M. Ni-based electrocatalyst for water oxidation developed in-situ in a HCO3–/CO2 system at near-neutral pH. Adv. Energy Mater.2014, 4, 1301929.
Zhang, H. Y.; Tian, W. J.; Li, Y. G.; Sun, H. Q.; Tadé, M. O.; Wang, S. B. A comparative study of metal (Ni, Co, or Mn)-borate catalysts and their photodeposition on rGO/ZnO nanoarrays for photoelectrochemical water splitting. J. Mater. Chem. A2018, 6, 24149–24156.
Man, I. C.; Su, H. Y.; Calle-Vallejo, F.; Hansen, H. A.; Martínez, J. I.; Inoglu, N. G.; Kitchin, J.; Jaramillo, T. F.; Nørskov, J. K.; Rossmeisl, J. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem2011, 3, 1159–1165.
Suntivich, J.; May, K. J.; Gasteiger, H. A.; Goodenough, J. B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science2011, 334, 1383–1385.
Subbaraman, R.; Tripkovic, D.; Chang, K. C.; Strmcnik, D.; Paulikas, A. P.; Hirunsit, P.; Chan, M.; Greeley, J.; Stamenkovic, V.; Markovic, N. M. Trends in activity for the water electrolyser reactions on 3d M (Ni, Co, Fe, Mn) hydr (oxy) oxide catalysts. Nat. Mater.2012, 11, 550–557.
Acknowledgements
This work is supported by the Basic Science Center Program for Ordered Energy Conversion of the National Natural Science Foundation of China (No. 51888103), the China National Key Research and Development Plan Project (No. 2018YFB1502000), and the Natural Science Basic Research Plan in Shaanxi Province of China (No. 2019JM-400).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2019_2605_MOESM1_ESM.pdf
Room-temperature photodeposition of conformal transition metal based cocatalysts on BiVO4 for enhanced photoelectrochemical water splitting
Rights and permissions
About this article
Cite this article
Wang, L., Zhang, T., Su, J. et al. Room-temperature photodeposition of conformal transition metal based cocatalysts on BiVO4 for enhanced photoelectrochemical water splitting. Nano Res. 13, 231–237 (2020). https://doi.org/10.1007/s12274-019-2605-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2605-3