Abstract
Purpose of Review
Obesity and obesity-related diseases, largely resulting from urbanization and behavioral changes, are now of global importance. Energy restriction, though, is associated with health improvements and increased longevity. We review some important mechanisms related to calorie limitation aimed at controlling of metabolic diseases, particularly diabetes.
Recent Findings
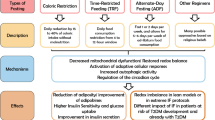
Calorie restriction triggers a complex series of intricate events, including activation of cellular stress response elements, improved autophagy, modification of apoptosis, and alteration in hormonal balance. Intermittent fasting is not only more acceptable to patients, but it also prevents some of the adverse effects of chronic calorie restriction, especially malnutrition.
Summary
There are many somatic and potentially psychologic benefits of fasting or intermittent calorie restriction. However, some behavioral modifications related to abstinence of binge eating following a fasting period are crucial in maintaining the desired favorable outcomes.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Badran M, Laher I. Obesity in arabic-speaking countries. J Obes. 2011;2011:686430. https://doi.org/10.1155/2011/686430.
Badran M, Laher I. Type II diabetes mellitus in Arabic-speaking countries. Int J Endocrinol. 2012;2012:902873. https://doi.org/10.1155/2012/902873.
• Abuyassin B, Laher I. Diabetes epidemic sweeping the Arab world. World J Diabetes. 2016;7:165–74. https://doi.org/10.4239/wjd.v7.i8.165. This review article gives an overview of epidemiologic aspects of diabetes, particularly in respect to unhealthy lifestyle, in the Middle East.
Abuyassin B, Laher I. Obesity-linked diabetes in the Arab world: a review. East Mediterr Health J. 2015;21:42039.
Trepanowski JF, Bloomer RJ. The impact of religious fasting on human health. Nutr J. 2010;9:57. https://doi.org/10.1186/1475-2891-9-57.
Persynaki A, Karras S, Pichard C. Unraveling the metabolic health benefits of fasting related to religious beliefs: a narrative review. Nutrition. 2017;35:14–20. https://doi.org/10.1016/j.nut.2016.10.005.
el Ati J, Beji C, Danguir J. Increased fat oxidation during Ramadan fasting in healthy women: an adaptative mechanism for body-weight maintenance. Am J Clin Nutr. 1995;62:302–7.
Al Suwaidi J, Bener A, Hajar HA, Numan MT. Does hospitalization for congestive heart failure occur more frequently in Ramadan: a population-based study [1991-2001]. Int J Cardiol. 2004;96:217–21.
Al Suwaidi J, Bener A, Suliman A, Hajar R, Salam AM, Numan MT, Al Binali HA. Al Suwaidi J, Bener A, Suliman A, Hajar R, Salam AM, Numan MT, Al Binali HA. A population based study of Ramadan fasting and acute coronary syndromes. Heart. 2004; 90: 695–696.
Al Suwaidi J, Bener A, Gehani AA, Behair S, Al Mohanadi D, Salam A, et al. Does the circadian pattern for acute cardiac events presentation vary with fasting? J Postgrad Med. 2006;52:30–3.
Bahijri S, Borai A, Ajabnoor G, Abdul Khaliq A, AlQassas I, Al-Shehri D, et al. Relative metabolic stability, but disrupted circadian cortisol secretion during the fasting month of Ramadan. PLoS One. 2013;8:e60917. https://doi.org/10.1371/journal.pone.0060917.
Charmandari ETC, Chrousos GP. Neuroendocrinology of stress. Annu Rev Physiol. 2005;67:259–84.
Pervanidou P, Chrousos GP. Metabolic consequences of stress during childhood and adolescence. Metabolism. 2012;61:611–9. https://doi.org/10.1016/j.metabol.2011.10.005.
Maislos M, Abou-Rabiah Y, Zuili I, Iordash S, Shany S. Gorging and plasma HDL-cholesterol—the Ramadan model. Eur J Clin Nutr. 1998;52:127–30.
Koh HK, Joossens LX, Connolly GN. Making smoking history worldwide. N Engl J Med. 2007;356:1496–8.
Ramahi I, Seidenberg AB, Kennedy RD, Rees VW. Secondhand smoke emission levels in enclosed public places during Ramadan. Eur J Public Health 2013; 789–91. doi: https://doi.org/10.1093/eurpub/cks119.
Thomas JA 2nd, Antonelli JA, Lloyd JC, Masko EM, Poulton SH, Phillips TE, et al. Effect of intermittent fasting on prostate cancer tumor growth in a mouse model. Prostate Cancer Prostatic Dis. 2010;13:350–5. https://doi.org/10.1038/pcan.2010.24.
Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–62.
Buschemeyer WC 3rd, Klink JC, Mavropoulos JC, Poulton SH, Demark-Wahnefried W, Hursting SD, et al. Effect of intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate. 2010;70:1037–43. https://doi.org/10.1002/pros.21136.
Ikeno Y, Lew CM, Cortez LA, Webb CR, Lee S, Hubbard GB. Do long-lived mutant and calorie-restricted mice share common anti-aging mechanisms? A pathological point of view. Age (Dordr). 2006;28:163–71. https://doi.org/10.1007/s11357-006-9007-7.
Maeda H, Gleiser CA, Masoro EJ, Murata I, McMahan CA, Yu BP. Nutritional influences on aging of Fischer 344 rats: II. Pathol J Gerontol. 1985;40:671–88.
Cava E, Fontana L. Will calorie restriction work in humans? Aging (Albany NY). 2013;5:507–14.
Mercken EM, Crosby SD, Lamming DW, JeBailey L, Krzysik-Walker S, Villareal DT, et al. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12:645–51. https://doi.org/10.1111/acel.12088.
Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48.
Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328 https://doi.org/10.1126/science.
Mattson MP. Challenging oneself intermittently to improve health. Dose Response. 2014;12:600–18. https://doi.org/10.2203/dose-response.14-028.Mattson. eCollection 2014
Adrie C, Richter C, Bachelet M, Banzet N, François D, Dinh-Xuan AT, et al. Contrasting effects of NO and peroxynitrites on HSP70 expression and apoptosis in human monocytes. Am J Physiol Cell Physiol. 2000;279:C452–60.
Guttman SD, Glover CV, Allis CD, Gorovsky MA. Heat shock, deciliation and release from anoxia induce the synthesis of the same set of polypeptides in starved T. pyriformis. Cell. 1980;22:299–307.
Chiang HL, Terlecky SR, Plant CP, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246:382–5.
Sciandra JJ, Subjeck JR. The effects of glucose on protein synthesis and thermosensitivity in Chinese hamster ovary cells. J Biol Chem. 1983;258:12091–3.
Morton JP, Kayani AC, McArdle A, Drust B. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med. 2009;39:643–62. https://doi.org/10.2165/00007256-200939080-00003.
Geiger PC, Gupte AA. Heat shock proteins are important mediators of skeletal muscle insulin sensitivity. Exerc Sport Sci Rev. 2011;39:34–42. https://doi.org/10.1097/JES.0b013e318201f236.
Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, et al. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51:1102–9.
Atalay M, Oksala N, Lappalainen J, Laaksonen DE, Sen CK, Roy S. Heat shock proteins in diabetes and wound healing. Curr Protein Pept Sci. 2009;10:85–9.
Bijur GN, Jope RS. Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3beta in the regulation of HSF-1 activity. J Neurochem. 2000;75:2401–8.
Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105:1739–44. https://doi.org/10.1073/pnas.0705799105.
Speakman JR, Mitchell SE. Caloric restriction. Mol Asp Med. 2011;32:159–221. https://doi.org/10.1016/j.mam.2011.07.001.
Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67:41–52. https://doi.org/10.1002/ana.21798.
Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. https://doi.org/10.1038/cddis.2009.8.
Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911–916. e12. https://doi.org/10.1016/j.jada.2010.03.018.
Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, et al. Orally absorbed reactive glycation products [glycotoxins] : an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci U S A. 1997;94:6474–9.
Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20.
Cai W, He JC, Zhu L, Chen X, Wallenstein S, Striker GE, et al. Reduced oxidant stress and extended lifespan in mice exposed to a low glycotoxin diet: association with increased AGER1 expression. Am J Pathol. 2007;170:1893–902.
Stern D, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation end-products: a multiligand receptor magnifying cell stress in diverse pathologic settings. Adv Drug Deliv Rev. 2002;54:1615–25.
Bierhaus A, Humpert PM, Stern DM, Arnold B, Nawroth PP. Advanced glycation end product receptor-mediated cellular dysfunction. Ann N Y Acad Sci. 2005;1043:676–80.
Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem. 1997;272:16498–506.
Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90.
Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742–52. https://doi.org/10.1681/ASN.2008050514.
Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–94.
Gugliucci A, Kotani K, Taing J, Matsuoka Y, Sano Y, Yoshimura M, et al. Short-term low calorie diet intervention reduces serum advanced glycation end products in healthy overweight or obese adults. Ann Nutr Metab. 2009;54:197–201. https://doi.org/10.1159/000217817.
Iwashige K, Kouda K, Kouda M, Horiuchi K, Takahashi M, Nagano A, et al. Calorie restricted diet and urinary pentosidine in patients with rheumatoid arthritis. J Physiol Anthropol Appl Hum Sci. 2004;23:19–24.
Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, et al. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–76.
Wan R, Ahmet I, Brown M, Cheng A, Kamimura N, Talan M, et al. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J Nutr Biochem. 2010;21:413–7. https://doi.org/10.1016/j.jnutbio.2009.01.020.
Mazaki-Tovi S, Kanety H, Sivan E. Adiponectin and human pregnancy. Curr Diab Rep. 2005;5:278–81.
Okamoto M, Ohara-Imaizumi M, Kubota N, Hashimoto S, Eto K, Kanno T, et al. Adiponectin induces insulin secretion in vitro and in vivo at a low glucose concentration. Diabetologia. 2008;51:827–35. https://doi.org/10.1007/s00125-008-0944-9.
Musso G, Gambino R, Biroli G, Carello M, Fagà E, Pacini G, et al. Hypoadiponectinemia predicts the severity of hepatic fibrosis and pancreatic Beta-cell dysfunction in nondiabetic nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2005;100:2438–46.
Retnakaran R, Hanley AJ, Raif N, Hirning CR, Connelly PW, Sermer M, et al. Adiponectin and beta cell dysfunction in gestational diabetes: pathophysiological implications. Diabetologia. 2005;48:993–1001.
Cui J, Panse S, Falkner B. The role of adiponectin in metabolic and vascular disease: a review. Clin Nephrol. 2011;75:26–33.
Bik W, Baranowska-Bik A, Wolinska-Witort E, Martynska L, Chmielowska M, Szybinska A, et al. The relationship between adiponectin levels and metabolic status in centenarian, early elderly, young and obese women. Neuro Endocrinol Lett. 2006;27:493–500.
Atzmon G, Pollin TI, Crandall J, Tanner K, Schechter CB, Scherer PE, et al. Adiponectin levels and genotype: a potential regulator of life span in humans. J Gerontol A Biol Sci Med Sci. 2008;63:447–53.
Klöting N, Blüher M. Extended longevity and insulin signaling in adipose tissue. Exp Gerontol. 2005;40:878–83.
Alderman JM, Flurkey K, Brooks NL, Naik SB, Gutierrez JM, Srinivas U, et al. Neuroendocrine inhibition of glucose production and resistance to cancer in dwarf mice. Exp Gerontol. 2009;44:26–33. https://doi.org/10.1016/j.exger.2008.05.014.
Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J Gerontol A Biol Sci Med Sci. 2006;61:323–31.
Qiao L, Lee B, Kinney B, Yoo HS, Shao J. Energy intake and adiponectin gene expression. Am J Physiol Endocrinol Metab. 2011;300:E809–16. https://doi.org/10.1152/ajpendo.00004.2011.
Nakamura T, Funayama H, Kubo N, Yasu T, Kawakami M, Saito M, et al. Association of hyperadiponectinemia with severity of ventricular dysfunction in congestive heart failure. Circ J. 2006;70:1557–62.
Nelson DL, Cox MM. Lehninger principles of biochemistry. 6th ed. New York: W.H. Freeman and Company; 2013.
Voet D, Voet JG. Biochemistry. 4th ed. Chichester: Wiley; 2011.
Weyer C, Foley EJ, Bogardus C, Tataranni AP, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–506.
Varady KA, Hellerstein MK. Do calorie restriction or alternate-day fasting regimens modulate adipose tissue physiology in a way that reduces chronic disease risk? Nutr Rev. 2008;66:333–42.
Varady KA, Roohk DJ, Loe YC, McEvoy-Hein BK, Hellerstein MK. Effects of modified alternate-day fasting regimens on adipocyte size, triglyceride metabolism and plasma adiponectin levels in mice. J Lipid Res. 2007;48:2212–9.
Tzur R, Rose-Kahn G, Adler HJ, Bar-Tana J. Hypolipidemic, antiobesity, and hypoglycemic-hypoinsulinemic effects of beta,beta’-methyl-substituted hexadecanedioic acid in sand rats. Diabetes. 1988;37:1618–24.
Varady AK, Hellerstein KM. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86:7–13.
Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes. 2011;35:714–27.
•• Ding H, Zheng S, Garcia-Ruiz D, Hou D, Wei Z, Liao Z, et al. Fasting induces a subcutaneous-to-visceral fat switch mediated by microRNA-149-3p and suppression of PRDM16. Nat Commun. 2016; https://doi.org/10.1038/ncomms11533. This study provides a novel view of adipose tissue metabolism during fasting and the importance of subcutaneous fat in energy balance.
• Fabbiano S, Suárez-Zamorano N, Rigo D, Veyrat-Durebex C, Stevanovic Dokic A, Colin DJ, et al. Caloric restriction leads to browning of white adipose tissue through type 2 immune signaling. Cell Metab. 2016;24:434–46. https://doi.org/10.1016/j.cmet.2016.07.023. An investigation of the metabolism of adipose tissue and its potential for transformation during periods of energy restriction
Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol. 2010;6:195–213.
McKnight JR, Satterfield MC, Jobgen WS, Smith SB, Spencer TE, Meininger CJ, et al. Beneficial effects of L-arginine on reducing obesity: potential mechanisms and important implications for human health. Amino Acids. 2010;39:349–57.
Otasevic V, Korac A, Buzadzic B, Stančić A, Janković A. Korać B. Nitric oxide and thermogenesis-challenge in molecular cell physiology. Front Biosci 2011; 3: 1180–1195.
Stanford IK, Middelbeek JWR, Goodyear JL. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes. 2015; https://doi.org/10.2337/db15-0227.
Romaniello, J: IF 201: a look at four popular intermittent fasting protocols. A breakdown of the most popular IF variations. URL romanfitnesssystems.com/articles/intermittent-fasting-201/.
Barnosky AR, Hoddy KK, Unterman TG, Varady KA. Intermittent fasting vs daily calorie restriction for type 2 diabetes prevention: a review of human findings. Transl Res. 2014;164:302–11. https://doi.org/10.1016/j.trsl.2014.05.013.
Varady KA. Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obes Rev. 2011;12:e593–601. https://doi.org/10.1111/j.1467-789X.2011.00873.x.
Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–20.
Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–4. https://doi.org/10.1126/science.1173635.
Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–7.
Clément K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–69.
Eshghinia S, Mohammadzadeh F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J Diabetes Metab Disord. 2013;12:1–4.
Klempel MC, Kroeger CM, Bhutani S, Trepanowski JF, Varady KA. Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutr J. 2012;11:98.
Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: a novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr. 2009;90:1138–43.
Klempel MC, Kroeger CM, Varady KA. Alternate day fasting [ADF] with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism. 2013;62:137–43.
Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in non-obese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005;81:69–73.
M'guil M, Ragala MA, El Guessabi L, Fellat S, Chraibi A, Chabraoui L, et al. Is Ramadan fasting safe in type 2 diabetic patients in view of the lack of significant effect of fasting on clinical and biochemical parameters, blood pressure, and glycemic control ? Clin Exp Hypertens. 2008;30:339–57. https://doi.org/10.1080/10641960802272442.
Deng X, Cheng J, Zhang Y, Li N, Chen L. Effects of caloric restriction on SIRT1 expression and apoptosis of islet beta cells in type 2 diabetic rats. Acta Diabetol. 2010;47(suppl 1):177–85. https://doi.org/10.1007/s00592-009-0159-7.
Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–8.
Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–67.
Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One. 2008;3:e1759. https://doi.org/10.1371/journal.pone.0001759.
Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, et al. CALERIE Pennington team. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76.
Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012;2012:941868. https://doi.org/10.1155/2012/941868.
Krumholz HM, Currie PM, Riegel B, Phillips CO, Peterson ED, Smith R, et al. A taxonomy for disease management: a scientific statement from the American Heart Association disease management taxonomy writing group. Circulation. 2006;114:1432–45.
Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev. 2009;89:27–71. https://doi.org/10.1152/physrev.00014.2008.
Yung LM, Leung FP, Yao X, Chen ZY, Huang Y. Reactive oxygen species in vascular wall. Cardiovasc Hematol Disord Drug Targets. 2006;6:1–19.
Meydani M, Das S, Band M, Epstein S, Roberts S. The effect of caloric restriction and glycemic load on measures of oxidative stress and antioxidants in humans: results from the CALERIE trial of human caloric restriction. J Nutr Health Aging. 2011;15:456–60.
Buchowski MS, Hongu N, Acra S, Wang L, Warolin J, Roberts LJ 2nd. Effect of modest caloric restriction on oxidative stress in women, a randomized trial. PLoS One. 2012;7:e47079. https://doi.org/10.1371/journal.pone.0047079.
Sung MM, Dyck JR. Age-related cardiovascular disease and the beneficial effects of calorie restriction. Heart Fail Rev. 2012;17:707–19. https://doi.org/10.1007/s10741-011-9293-8.
Han X, Ren J. Caloric restriction and heart function: is there a sensible link? Acta Pharma. 2010;31:1111–7.
Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–60.
Seymour EM, Parikh RV, Singer AA, Bolling SF. Moderate calorie restriction improves cardiac remodeling and diastolic dysfunction in the Dahl-SS rat. J Mol Cell Cardiol. 2006;41:661–8.
Zotova AV, Desyatova IE, Bychenko SM, Sivertseva SA, Okonechnikova NS, Murav'ev SA. The efficacy of low calorie diet therapy in patients with arterial hypertension and chronic cerebral ischemia. Zh Nevrol Psikhiatr Im S S Korsakova. 2015;115:25–8.
Chatterjee A, Black SM, Catravas JD. Endothelial nitric oxide [NO] and its pathophysiologic regulation. Vasc Pharmacol. 2008;49:134–40.
Karbach S, Wenzel P, Waisman A, Munzel T, Daiber A. eNOS uncoupling in cardiovascular diseases—the role of oxidative stress and inflammation. Curr Pharm Des. 2014;20:3579–94.
Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–37.
Weiss EP, Fontana L. Caloric restriction: powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol. 2011;301:H1205–19. https://doi.org/10.1152/ajpheart.00685.2011.
Horne BD, Muhlestein JB, Anderson JL. Health effects of intermittent fasting: hormesis or harm? A systematic review. Am J Clin Nutr. 2015;102:464–70. https://doi.org/10.3945/ajcn.115.109553.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Saeid Golbidi, Andreas Daiber, Bato Korac, Huige Li, M. Faadiel Essop, and Ismail Laher declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Lifestyle Management to Reduce Diabetes/Cardiovascular Risk
Rights and permissions
About this article
Cite this article
Golbidi, S., Daiber, A., Korac, B. et al. Health Benefits of Fasting and Caloric Restriction. Curr Diab Rep 17, 123 (2017). https://doi.org/10.1007/s11892-017-0951-7
Published:
DOI: https://doi.org/10.1007/s11892-017-0951-7