Abstract
Objective
To explore the possible underlying mechanism by investigating the effect of electroacupuncture (EA) treatment on the primary motor cortex and striatum in a unilateral 6-hydroxydopamine (6-OHDA) induced rat Parkinson’s disease (PD) model.
Methods
Male Sprague-Dawley rats were randomly divided into sham group (n=16), model group (n=14), and EA group (n=14). EA stimulation at Dazhui (GV 14) and Baihui (GV20) was applied to PD rats in the EA group for 4 weeks. Behavioral tests were conducted to evaluate the effectiveness of EA treatment. Metabolites were detected by 7.0 T proton nuclear magnetic resonance.
Results
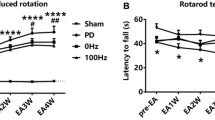
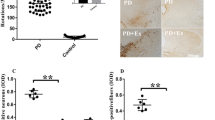
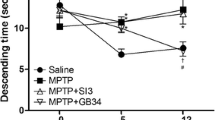
Following 4 weeks of EA treatment in PD model rats, the abnormal behavioral impairment induced by 6-OHDA was alleviated. In monitoring changes in metabolic activity, ratios of myoinositol/creatine (Cr) and N-acetyl aspartate (NAA)/Cr in the primary motor cortex were significantly lower at the injected side than the non-injected side in PD rats (P=0.024 and 0.020). The ratios of glutamate + glutamine (Glx)/Cr and NAA/Cr in the striatum were higher and lower, respectively, at the injected side than the non-injected side (P=0.046 and 0.008). EA treatment restored the balance of metabolic activity in the primary motor cortex and striatum. In addition, the taurine/Cr ratio and Glx/Cr ratio were elevated in the striatum of PD model rats compared to sham-lesioned rats (P=0.026 and 0.000). EA treatment alleviated the excessive glutamatergic transmission by down-regulating the striatal Glx/Cr ratio (P=0.001). The Glx/Cr ratio was negatively correlated with floor plane spontaneous locomotion in PD rats (P=0.027 and P=0.0007).
Conclusions
EA treatment is able to normalize the metabolic balance in the primary motor cortex and striatum of PD rats, which may contribute to its therapeutic effect on motor deficits. The striatal Glx/Cr ratio may serve as a potential indicator of PD and a therapeutic target of EA treatment.
Similar content being viewed by others
References
Grayson M. Parkinson’s disease. Nature 2016;538:S1.
Lindenbach D, Bishop C. Critical involvement of the motor cortex in the pathophysiology and treatment of Parkinson’s disease. Neurosci Biobehav Rev 2013;37:2737–2750.
Lee JY, Kim SH, Ko AR, Lee JS, Yu JH, Seo JH, et al. Therapeutic effects of repetitive transcranial magnetic stimulation in an animal model of Parkinson’s disease. Brain Res 2013;1537:290–302.
Li Q, Ke Y, Chan DC, Qian ZM, Yung KK, Ko H, et al. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron 2012;76:1030–1041.
Yang YR, Tseng CY, Chiou SY, Liao KK, Cheng SJ, Lai KL, et al. Combination of rTMS and treadmill training modulates corticomotor inhibition and improves walking in Parkinson disease: a randomized trial. Neurorehabil Neural Repair 2013;27:79–86.
Ciurleo R, Di Lorenzo G, Bramanti P, Marino S. Magnetic resonance spectroscopy: an in vivo molecular imaging biomarker for Parkinson’s disease? Biomed Res Int 2014;2014:519816.
Lucetti C, Del Dotto P, Gambaccini G, Bernardini S, Bianchi MC, Tosetti M, et al. Proton magnetic resonance spectroscopy (1H-MRS) of motor cortex and basal ganglia in de novo Parkinson’s disease patients. Neurol Sci 2001;22:69–70.
Lucetti C, Del Dotto P, Gambaccini G, Ceravolo R, Logi C, Berti C, et al. Influences of dopaminergic treatment on motor cortex in Parkinson disease: a MRI/MRS study. Mov Disord 2007;22:2170–2175.
Melon C, Chassain C, Bielicki G, Renou JP, Kerkerian-Le Goff L, Salin P, et al. Progressive brain metabolic changes under deep brain stimulation of subthalamic nucleus in parkinsonian rats. J Neurochem 2015;132:703–712.
Bagga P, Chugani AN, Varadarajan KS, Patel AB. In vivo NMR studies of regional cerebral energetics in MPTP model of Parkinson’s disease: recovery of cerebral metabolism with acute levodopa treatment. J Neurochem 2013;127:365–377.
Chassain C, Bielicki G, Keller C, Renou JP, Durif F. Metabolic changes detected in vivo by 1H MRS in the MPTP-intoxicated mouse. NMR Biomed 2010;23:547–553.
Chassain C, Bielicki G, Durand E, Lolignier S, Essafi F, Traore A, et al. Metabolic changes detected by proton magnetic resonance spectroscopy in vivo and in vitro in a murin model of Parkinson’s disease, the MPTP-intoxicated mouse. J Neurochem 2008;105:874–882.
Modrego PJ, Fayed N, Artal J, Olmos S. Correlation of findings in advanced MRI techniques with global severity scales in patients with Parkinson disease. Acad Radiol 2011;18:235–241.
Coune PG, Craveiro M, Gaugler MN, Mlynarik V, Schneider BL, Aebischer P, et al. An in vivo ultrahigh field 14.1 T 1H-MRS study on 6-OHDA and alpha-synuclein-based rat models of Parkinson’s disease: GABA as an early disease marker. NMR Biomed 2013;26:43–50.
Abe K, Terakawa H, Takanashi M, Watanabe Y, Tanaka H, Fujita N, et al. Proton magnetic resonance spectroscopy of patients with parkinsonism. Brain Res Bull 2000;52:589–595.
Arankalle DV, Nair PM. Effect of electroacupuncture on function and quality of life in Parkinson’s disease: a case report. Acupunct Med 2013;31:235–238.
Xiao D. Acupuncture for Parkinson’s disease: a review of clinical, animal, and functional magnetic resonance imaging studies. J Tradit Chin Med 2015;35:709–717.
Wang F, Sun L, Zhang XZ, Jia J, Liu Z, Huang XY, et al. Effect and potential mechanism of electroacupuncture addon treatment in patients with Parkinson’s disease. Evid Based Complement Alternat Med 2015;2015:692795.
Lv E, Deng J, Yu Y, Wang Y, Gong X, Jia J, et al. Nrf2-ARE signals mediated the anti-oxidative action of electroacupuncture in an MPTP mouse model of Parkinson’s disease. Free Radic Res 2015;49:1296–1307.
Deng J, Lv E, Yang J, Gong X, Zhang W, Liang X, et al. Electroacupuncture remediates glial dysfunction and ameliorates neurodegeneration in the astrocytic alpha-synuclein mutant mouse model. J Neuroinflammation 2015;12:103.
Yu Y, Wang K, Deng J, Sun M, Jia J, Wang X. Electroacupuncture produces the sustained motor improvement in 6-hydroxydopamine-lesioned mice. PLoS One 2016;11:e0149111.
Huo LR, Liang XB, Li B, Liang JT, He Y, Jia YJ, et al. The cortical and striatal gene expression profile of 100 hz electroacupuncture treatment in 6-hydroxydopamineinduced Parkinson’s disease model. Evid Based Complement Alternat Med 2012;2012:908439.
Jia YJ, Deng JH, Zhang WZ, Sun ZL, Yang J, Yu Y, et al. The role of group II metabotropic glutamate receptors in the striatum in electroacupuncture treatment of Parkinsonian rats. CNS Neurosci Ther 2017;23:23–32.
Jia J, Sun Z, Li B, Pan Y, Wang H, Wang X, et al. Electroacupuncture stimulation improves motor disorders in Parkinsonian rats. Behav Brain Res 2009;205:214–218.
Sun Z, Jia J, Gong X, Jia Y, Deng J, Wang X. Inhibition of glutamate and acetylcholine release in behavioral improvement induced by electroacupuncture in parkinsonian rats. Neurosci Lett 2012;520:32–37.
Choi CB, Kim SY, Lee SH, Jahng GH, Kim HY, Choe BY, et al. Assessment of metabolic changes in the striatum of a MPTP-intoxicated canine model: in vivo1H-MRS study of an animal model for Parkinson’s disease. Magn Reson Imaging 2011;29:32–39.
Griffith HR, Okonkwo OC, O’Brien T, Hollander JA. Reduced brain glutamate in patients with Parkinson’s disease. NMR Biomed 2008;21:381–387.
Fan XT, Zhao F, Ai Y, Andersen A, Hardy P, Ling F, et al. Cortical glutamate levels decrease in a non-human primate model of dopamine deficiency. Brain Res 2014;1552:34–40.
Choe BY, Park JW, Lee KS, Son BC, Kim MC, Kim BS, et al. Neuronal laterality in Parkinson’s disease with unilateral symptom by in vivo1H magnetic resonance spectroscopy. Invest Radiol 1998;33:450–455.
Patki G, Che Y, Lau YS. Mitochondrial dysfunction in the striatum of aged chronic mouse model of Parkinson’s disease. Front Aging Neurosci 2009;1:3.
Subramaniam SR, Vergnes L, Franich NR, Reue K, Chesselet MF. Region specific mitochondrial impairment in mice with widespread overexpression of alpha-synuclein. Neurobiol Dis 2014;70:204–213.
Wang H, Pan Y, Xue B, Wang X, Zhao F, Jia J, et al. The antioxidative effect of electro-acupuncture in a mouse model of Parkinson’s disease. PLoS One 2011;6:e19790.
Boulet S, Mounayar S, Poupard A, Bertrand A, Jan C, Pessiglione M, et al. Behavioral recovery in MPTPtreated monkeys: neurochemical mechanisms studied by intrastriatal microdialysis. J Neurosci 2008;28:9575–9584.
Hornykiewicz O. Chemical neuroanatomy of the basal ganglia—normal and in Parkinson’s disease. J Chem Neuroanat 2001;22:3–12.
Gao HC, Zhu H, Song CY, Lin L, Xiang Y, Yan ZH, et al. Metabolic changes detected by ex vivo high resolution 1H NMR spectroscopy in the striatum of 6-OHDA-induced Parkinson’s rat. Mol Neurobiol 2013;47:123–130.
Chepkova AN, Sergeeva OA, Haas HL. Long-lasting enhancement of corticostriatal transmission by taurine: role of dopamine and acetylcholine. Cell Mol Neurobiol 2005;25:767–776.
Acknowledgements
Authors thank Dr. WANG Qiang (University of Missouri Kansas City) for his critical readings of the paper.
Author information
Authors and Affiliations
Contributions
Li M and Wang XM designed the study. Li M and Su WT performed the experiments. Li M and Wang K wrote the paper. Jia J and Wang XM reviewed and edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Additional information
Conflict of Interest
The authors declared that they have no conflicts of interest to this work.
Supported by the National Natural Science Foundation of China (No. 81072858, 81473770, and 81030062), Beijing Municipal Science and Technology Commission (No. Z161100002616007)
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, M., Wang, K., Su, Wt. et al. Effects of Electroacupuncture on Metabolic Changes in Motor Cortex and Striatum of 6-Hydroxydopamine-Induced Parkinsonian Rats. Chin. J. Integr. Med. 26, 701–708 (2020). https://doi.org/10.1007/s11655-017-2975-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-017-2975-x