Abstract
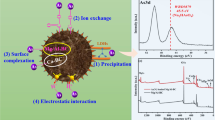
The pollution of toxic and persistent heavy metals commonly exist in water environment; such multi-component pollutants pose a serious threat to human beings and other organisms. Herein, to make full use of the advantages of both layered double hydroxide (LDH) and rice husk ash (RHA), a novel Mg–Fe-LDH-RHA functional material was synthesized by assembling LDH on the biochar derived from RHA and used as an adsorbent for removal of heavy metal ions including Pb2+, Cu2+, Co2+, Ni2+, Zn2+, and Cd2+. The adsorption kinetics and isotherms of heavy metal ions in a mono-component system, the adsorption capacities in mixed multi-metal ion system, and the regeneration of the adsorbent were studied in detail. The results showed that the synthesized Mg–Fe-LDH-RHA might efficiently remove the above six heavy metals in water under optimized experimental conditions. Interestingly, the removal performance toward Pb(II) showed high static distribution coefficients (Kd) of ~ 107 mL/g and maximum capacity of ~ 682 mg/g. Besides, further characterizations of the adsorbent have been conducted, and the result suggested the formation of abundant functional groups including hydroxyl, carbonyl, and carboxyl groups. The removal mechanism of the metal ions might be related to ion-exchange, surface precipitation, complexation, and hydrogen binding during the interactions between the LDH-RHA material and pollutants. Such a facile and environmentally friendly approach, efficient removal performance suggests that the LDH-RHA material thus has potential for efficient removal of heavy metals in practical application.





Similar content being viewed by others
References
Abukhadra MR, Dardir FM, Shaban M, Ahmed EA, Soliman MF (2018) Superior removal of Co2+, Cu2+ and Zn2+ contaminants from water utilizing spongy Ni/Fe carbonate–fluorapatite; preparation, application and mechanism. Ecotoxicol Environ Saf 157:358–368
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–33
Ahmet G, Ertan A, Ismail T (2007) Lead removal from aqueous solution by natural and pretreated clinoptilolite: adsorption equilibrium and kinetics. J Hazard Mater 146:362–371
Ali J, Wang HB, Ifthikar J, Khan A, Wang T, Zhan K, Shahzad A, Chen ZL, Chen ZQ (2018) Efficient, stable and selective adsorption of heavy metals by thio-functionalized layered double hydroxide in diverse types of water. Chem Eng J 332:387–397
Allison JD, Brown DS, Novo-Gradac KJ (1991) MINTEQA2-PRODEFA2, A Geochemical Assessment Model for Environmental Systems: Version 3.0. U.S. E.P.A., Athens, GA, USA
Chen B, Zhu ZL, Liu SX, Hong J, Ma J, Qiu YL, Chen JH (2014) Facile hydrothermal synthesis of nanostructured hollow iron-cerium alkoxides and their superior arsenic adsorption performance. ACS Appl Mater Interfaces 6:14016–14025
Ding ZH, Hu X, Zimmerman AR, Gao B (2014) Sorption and cosorption of lead (II) and methylene blue on chemically modified biomass. Bioresour Technol 167:569–573
Fan G, Li F, Evans DG, Duan X (2014) Catalytic applications of layered double hydroxides: recent advances and perspectives and as a consequence exhibit excellent expanding properties. Chem Soc Rev 43:7040–7066
Fan C, Li K, Li J, Ying D, Wang Y, Jia J (2017) Comparative and competitive adsorption of Pb(II) and Cu(II) using tetraethylenepentamine modified chitosan/CoFe2O4 particles. J Hazard Mater 326:211–220
Goh KH, Lim TT, Dong ZL (2008) Application of layered double hydroxides for removal of oxyanions: a review. Water Res 42:1343–1368
Gui CX, Wang QQ, Hao SM, Qu J, Huang PP, Cao CY, Song WG, Yu ZZ (2014) Sandwichlike magnesium silicate/reduced graphene oxide nanocomposite for enhanced Pb2+ and methylene blue adsorption. ACS Appl Mater Interfaces 6:14653–14659
Hao L, Song HJ, Zhang LC, Wan XY, Tang YR, Lv Y (2012) SiO2/graphene composite for highly selective adsorption of Pb(II) ion. J Colloid Interface Sci 369:381–387
Hayes KF, Leckie JO (1987) Modeling ionic strength effects on cation adsorption at hydrous oxide/solution interfaces. J Colloid Interface Sci 115:564–572
Hernandez-Montoya V, Perez-Cruz MA, Mendoza-Castillo DI, Moreno-Virgen MR, Bonilla-Petriciolet A (2013) Competitive adsorption of dyes and heavy metals on zeolitic structures. J Environ Manag 116:213–221
Hudcová B, Veselská V, Filip J, Číhalová S, Komárek M (2017) Sorption mechanism of arsenate on Mg-Fe layered double hydroxides: a combination of adsorption modeling and solid-state analysis. Chemosphere 168:539–548
Inyang M, Gao B, Yao Y, Xue Y, Zimmerman AR, Pullammanappallil P, Cao XD (2012) Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour Technol 110:50–56
Jaymand M (2014) Conductive polymers/zeolite (nano-) composites: under-exploited materials. RSC Adv 4:33935–33954
Kou L, Gao C (2011) Making silica nanoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nanoscale 3:519–528
Kwapinski W, Byrne CMP, Kryachko E, Wolfram P, Adley C, Leahy JJ, Novotny EH, Hayes MHB (2010) Biochar from biomass and waste. Waste Biomass Valor 1:177–189
Lee Y, Choi JH, Jeon HJ, Choi KM, Lee JW, Kang JK (2011) Titanium-embedded layered double hydroxides as highly efficient water oxidation photocatalysts under visible light. Energy Environ Sci 4:914–920
Li SJ, Zhang T, Li JF, Shi LN, Zhu XX, Lü JH, Li YM (2017) Stabilization of Pb(II) accumulated in biomass through phosphate-pretreated pyrolysis at low temperatures. J Hazard Mater 324:464–471
Li ZT, Wang L, Meng J, Liu XM, Xu JM, Wang F, Brookes P (2018) Zeolite-supported nanoscale zero-valent iron: new findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. J Hazard Mater 344:1–11
Ling L, Liu W-J, Zhang S, Jiang H (2016) Achieving high-efficiency and ultrafast removal of Pb(II) by one-pot incorporation of a N-doped carbon hydrogel into FeMg layered double hydroxides. J Mater Chem A 4:10336–10344
Liu M, Chen C, Hu J, Wu X, Wang X (2011a) Synthesis of magnetite/graphene oxide composite and application for cobalt (II) removal. J Phys Chem C 115:25234–25240
Liu WJ, Zeng FX, Jiang H, Yu HQ (2011b) pH-dependent interactions between lead and typha angustifolia biomass in the biosorption process. Ind Eng Chem Res 50:5920–5926
Liu W, Jiang H, Yu HQ (2015) Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev 115:12251–12285
Lu HL, Zhang WH, Yang YX, Huang XF, Wang SZ, Qiu RL (2012) Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Wat Res 46:854–862
Luo HP, Liu GL, Zhang RD, Bai YP, Fu SY, Hou YP (2014) Heavy metal recovery combined with H2 production from artificial acid mine drainage using the microbial electrolysis cell. J Hazard Mater 270:153–159
Lv L, He J, Wei M, Evans DG, Zhou Z (2007) Treatment of high fluoride concentration water by MgAl-CO3 layered double hydroxides: kinetic and equilibrium studies. Water Res 41:1534–1542
Ma L, Wang Q, Islam SM, Liu Y, Ma S, Kanatzidis MG (2016) Highly selective and efficient removal of heavy metals by layered double hydroxide intercalated with the MoS4 2− ion. J Am Chem Soc 138:2858–2866
Meng J, Feng XL, Dai ZM, Liu XM, Wu JJ, Xu J (2014) Adsorption characteristics of Cu(II) from aqueous solution onto biochar derived from swine manure. Environ Sci Pollut Res 21:7035–7046
Pode R (2016) Potential applications of rice husk ash waste from rice husk biomass power plant. Renew Sust Energ Rev 53:1468–1485
Rives V, del-Arco M, Martín C (2014) Intercalation of drugs in layered double hydroxides and their controlled release: a review. Appl Clay Sci 88-89:239–269
Romero-Dondiz EM, Almazan JE, Rajal VB, Castro-Vidaurre EF (2016) Comparison of the performance of ultrafiltration and nanofiltration membranes for recovery and recycle of tannins in the leather industry. J Clean Prod 135:71–79
Salima A, Benaouda B, Noureddine B, Duclaux L (2013) Application of Ulva lactuca and Systoceira stricta algae-based activated carbons to hazardous cationic dyes removal from industrial effluents. Water Res 47:3375–3388
Shaban M, Abukhadra MR (2017) Geochemical evaluation and environmental application of Yemeni natural zeolite as sorbent for Cd2+ from solution: kinetic modeling, equilibrium studies, and statistical optimization. Environ Earth Sci 76:310
Shaban M, Abukhadra MR, Khan AAP, Jibali BM (2018) Removal of Congo red, methylene blue and Cr(VI) ions from water using natural serpentine. J Taiwan Inst Chem Eng 82:102–116
Shaban M, Hassouna MEM, Nasief FM, Abukhadra MR (2017) Adsorption properties of kaolinite-based nanocomposites for Fe and Mn pollutants from aqueous solutions and raw ground water: kinetics and equilibrium studies. Environ Sci Pollut Res 24:22954–22966
Shen YF, Zhao PT, Shao QF (2014) Porous silica and carbon derived materials from rice husk pyrolysis char. Microporous Mesoporous Mater 188:46–76
Suman M, Kalzang C, Khaiwal R (2016) Application of agro-waste rice husk ash for the removal of phosphate from the wastewater. J Clean Prod 129:673–680
Wang Q, O’Hare D (2012) Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem Rev 112:4124–4155
Weng Y, Jiang B, Yang K (2015) Polyethyleneimine-modified graphene oxide nanocomposites for effective protein functionalization. Nanoscale 7:14284–14291
Yang SB, Hu J, Chen CL, Shao DD, Wang XK (2011) Mutual effect of Pb(II) and humic acid adsorption onto multiwalled carbon nanotubes/polyacrylamide composites from aqueous solution. Environ Sci Technol 45:3621–3627
Zhang M, Gao B, Fang J, Creamer AE, Ullman JL (2014) Self-assembly of needle-like layered double hydroxide (LDH) nanocrystals on hydrochar: characterization and phosphate removal ability. RSC Adv 4:28171–28175
Zhang F, Song Y, Song S, Zhang R, Hou W (2015) Synthesis of magnetite-graphene oxide-layered double hydroxide composites and applications for the removal of Pb(II) and 2,4-dichlorophenoxyacetic acid from aqueous solutions. ACS Appl Mater Interfaces 7:7251–7263
Zhao J, Chen J, Xu S, Shao M, Yan D, Wei M, Evans DG, Duan X (2013a) CoMn-layered double hydroxide nanowalls supported on carbon fibers for high-performance flexible energy storage devices. J Mater Chem A 1:8836–8843
Zhao F, Repo E, Yin D, Sillanpää MET (2013b) Adsorption of Cd(II) and Pb(II) by a novel EGTA-modified chitosan material: kinetics and isotherms. J Colloid Interface Sci 409:174–182
Zhao FP, Repo E, Yin DL, Meng Y, Jafari S, Sillanpää M (2015) EDTA-cross-linked β-cyclodextrin: an environmentally friendly bifunctional adsorbent for simultaneous adsorption of metals and cationic dyes. Environ Sci Technol 49:10570–10580
Zhao JH, Liu J, Li N, Wang W, Nan J, Zhao ZW, Cui FY (2016) Highly efficient removal of bivalent heavy metals from aqueous systems by magnetic porous Fe3O4-MnO2: adsorption behavior and process study. Chem Eng J 304:737–746
Zhou HG, Jiang ZM, Wei SQ (2018) A new hydrotalcite-like absorbent FeMnMg-LDH and its adsorption capacity for Pb2+ ions in water. Appl Clay Sci 153:29–37
Acknowledgements
This work was supported by the National Science and Technology Major Project of China (Grant No. 2017ZX07201005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Electronic supplementary material
ESM 1
(DOCX 66426 kb)
Rights and permissions
About this article
Cite this article
Yu, J., Zhu, Z., Zhang, H. et al. Mg–Fe layered double hydroxide assembled on biochar derived from rice husk ash: facile synthesis and application in efficient removal of heavy metals. Environ Sci Pollut Res 25, 24293–24304 (2018). https://doi.org/10.1007/s11356-018-2500-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2500-6