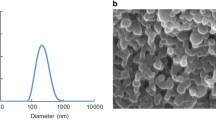
Abstract
Purpose
Polymeric microspheres are extensively researched for applications in drug and vaccine delivery. However, upon administration into the body, microspheres are primarily cleared via phagocytosis by macrophages. Although numerous studies have reported on the biochemical pathways of phagocytosis, relatively little is known about the dependence of phagocytosis on particle size. Here, we investigate the previously unexplained dependence of phagocytosis on particle size.
Methods
Rat alveolar macrophages and IgG-opsonized and non-opsonized polystyrene microspheres were used as model macrophages and drug delivery particles. Phagocytosis, attachment and internalization were measured by flow cytometry and time-lapse video microscopy.
Results
Particles possessing diameters of 2–3 μm exhibited maximal phagocytosis and attachment. Rate of internalization, however, was not affected significantly by particle size. Maximal attachment of 2–3 μm microspheres is hypothesized to originate from the characteristic features of membrane ruffles in macrophages. Elimination of ruffles via osmotic swelling nearly eliminated the peculiar size-dependence of phagocytosis. A simple mathematical model is presented to describe the dependence of phagocytosis on particle size.
Conclusions
The dependence of phagocytosis on particle size originated primarily from the attachment step. These results reveal the importance of controlling drug delivery particle size distribution and selecting the size appropriate for avoiding or encouraging phagocytosis.





Similar content being viewed by others
References
D. A. LaVan, D. M. Lynn, and R. Langer. Moving smaller in drug discovery and delivery. Nat. Rev. Drug Discov. 1:77–84 (2002).
J. Hanes, M. Chiba, and R. Langer. Polymer microspheres for vaccine delivery. Pharm. Biotechnol. 6:389–412 (1995).
V. R. Sinha, and A. Trehan. Biodegradable microspheres for protein delivery. J. Control. Release. 90:261–280 (2003).
C. X. Song, V. Labhasetwar, X. M. Cui, T. Underwood, and R. J. Levy. Arterial uptake of biodegradable nanoparticles for intravascular local drug delivery: Results with an acute dog model. J. Control. Release. 54:201–211 (1998).
M. Sakagami, and P. R. Byron. Respirable microspheres for inhalation: The potential of manipulating pulmonary disposition for improved therapeutic efficacy. Clin. Pharmacokinet. 44:263–277 (2005).
Y. Li, H. L. Jiang, K. J. Zhu, J. H. Liu, and Y. L. Hao. Preparation, characterization and nasal delivery of alpha-cobrotoxin-loaded poly(lactide-co-glycolide)/polyanhydride microspheres. J. Control. Release. 108:10–20 (2005).
Y. Yamaguchi, M. Takenaga, A. Kitagawa, Y. Ogawa, Y. Mizushima, and R. Igarashi. Insulin-loaded biodegradable PLGA microcapsules: Initial burst release controlled by hydrophilic additives. J. Control. Release. 81:235–249 (2002).
S. H. Djaldetti, M. Bergman, R. Djaldetti, and H. Bessler. Phagocytosis—the mighty weapon of the silent warriors. Microsc. Res. Tech. 57:421–431 (2002).
A. Aderem, and D. M. Underhill. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593–623 (1999).
S. D. Xiang, A. Scholzen, G. Minigo, C. David, V. Apostolopoulos, P. L. Mottram, and M. Plebanski. Pathogen recognition and development of particulate vaccines: Does size matter? Methods. 40:1–9 (2006).
L. Peiser, S. Mukhopadhyay, and S. Gordon. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 14:123–128 (2002).
S. P. Hart, J. R. Smith, and I. Dransfield. Phagocytosis of opsonized apoptotic cells: roles for 'old-fashioned' receptors for antibody and complement. Clin. Exp. Immunol. 135:181–185 (2004).
S. Greenberg, and S. Grinstein. Phagocytosis and innate immunity. Curr. Opin. Immunol. 14:136–145 (2002).
L. -A. H. Allen, and A. Aderem. Mechanisms of phagocytosis. Curr. Opin. Immunol. 8:36–40 (1996).
R. May, E. Caron, A. Hall, and L. M. Machesky. Involvement of the Arp2/3 complex in phagocytosis mediated by FcgammaR or CR3. Nat. Cell Biol. 2:246–248 (2000).
G. Kaplan. Differences in the mode of phagocytosis with Fc and C3 receptors in macrophages. Scand. J. Immunol. 6:797–807 (1977).
L. Kobzik. Lung macrophage uptake of unopsonized environmental particulates—role of scavenger-type receptors. J. Immunol. 155:367–376 (1995).
K. A. Beningo, and Y. L. Wang. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J. Cell Sci. 115:849–856 (2002).
J. A. Champion, and S. Mitragotri. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. U. S. A. 103:4930–4934 (2006).
F. L. Ahsan, I. P. Rivas, M. A. Khan, and A. I. T. Suarez. Targeting to macrophages: Role of physicochemical properties of particulate carriers—liposomes and microspheres—on the phagocytosis by macrophages. J. Control. Release. 79:29–40 (2002).
D. E. Owens III, and N. A. Peppas. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 307:93–102 (2006).
H. Kawaguchi, N. Koiwai, Y. Ohtsuka, M. Miyamoto, and S. Sasakawa. Phagocytosis of latex-particles by leukocytes .1. Dependence of phagocytosis on the size and surface-potential of particles. Biomaterials. 7:61–66 (1986).
Y. Tabata, and Y. Ikada. Effect of the size and surface-charge of polymer microspheres on their phagocytosis by macrophage. Biomaterials. 9:356–362 (1988).
S. I. Simon, and G. W. Schmidschonbein. Biophysical aspects of microsphere engulfment by human-neutrophils. Biophys. J. 53:163–173 (1988).
Y. Tabata, and Y. Ikada. Phagocytosis of polymer microspheres by macrophages. Adv. Polym. Sci. 94:107–141 (1990).
S. Rudt, and R. H. Muller. In vitro phagocytosis assay of nano- and microparticles by chemiluminescence III Uptake of differently sized surface-modified particles, and its correlation to particle properties and in vivo distribution. Eur. J. Pharm. Sci. 1:31–39 (1993).
H. M. Chen, R. Langer, and D. A. Edwards. A film tension theory of phagocytosis. J. Colloid Interface Sci. 190:118–133 (1997).
R. J. Helmke, V. F. German, and J. A. Mangos. A continuous alveolar macrophage cell-line—comparisons with freshly derived alveolar macrophages. In Vitro Cell Dev. Biol. Anim. 25:44–48 (1989).
J. A. Steinkamp, J. S. Wilson, G. C. Saunders, and C. C. Stewart. Phagocytosis—flow cytometric quantitation with fluorescent microspheres. Science. 215:64–66 (1982).
F. Schroeder, and D. A. Kinden. Measurement of phagocytosis using fluorescent latex beads. J. Biochem. Biophys. Methods. 8:15–27 (1983).
A. Palecanda, J. Paulauskis, E. Al-Mutairi, A. Imrich, G. Z. Qin, H. Suzuki, T. Kodama, K. Tryggvason, H. Koziel, and L. Kobzik. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J. Exp. Med. 189:1497–1506 (1999).
G. J. Dougherty, and W. H. McBride. Macrophage Heterogeneity. J. Clin. Lab. Immun. 14:1–11 (1984).
I. Carr. The Macrophage: A Review of Ultrastructure and Function. Academic Press, London, 1973.
D. Cox, P. Chang, Q. Zhang, P. G. Reddy, G. M. Bokoch, and S. Greenberg. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J. Exp. Med. 186:1487–1494 (1997).
M. M. Myat, S. Anderson, L. A. H. Allen, and A. Aderem. MARCKS regulates membrane ruffling and cell spreading. Curr. Biol. 7:611–614 (1997).
J. Israelchvili. Intermolecular and Surface Forces. Academic Press, San Diego, 1992.
E. M. V. Hoek, and G. K. Agarwal. Extended DLVO interactions between spherical particles and rough surfaces. J. Colloid Interface Sci. 298:50–58 (2006).
W. J. Kowalski, W. P. Bahnfleth, and T. S. Whittam. Filtration of airborne microoorganisms: modeling and prediction. ASHRAE Trans. 105:4–17 (1999).
G. W. Burnett, and G. S. Schuster. Pathogenic microbiology. The C.V. Mosby Co., St. Louis, 1973.
Acknowledgements
JAC was supported by a fellowship from the National Science Foundation. This work was supported by a grant from the University of California Biotechnology Research and Education Program. This work was partially supported by the MRSEC Program of the National Science Foundation under Award No. DMR00-80034.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Champion, J.A., Walker, A. & Mitragotri, S. Role of Particle Size in Phagocytosis of Polymeric Microspheres. Pharm Res 25, 1815–1821 (2008). https://doi.org/10.1007/s11095-008-9562-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9562-y